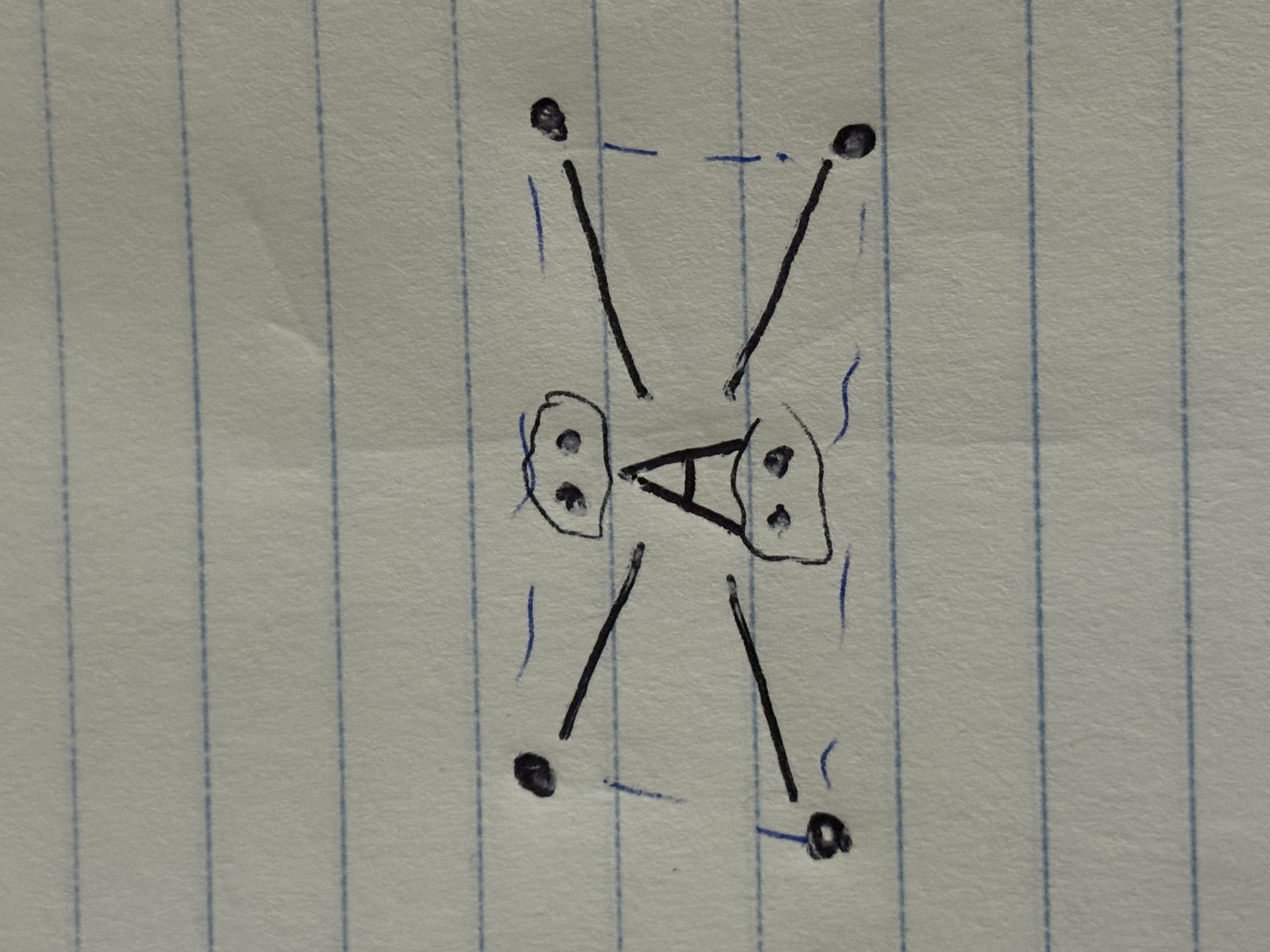
Electron Domain / Molecular Geometry
1/21
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
22 Terms
Domain
a bond (single, double, triple count as 1), or lone pair
In the equatorial area
When replacing bonding domains w/ nonbonding domains (lone pairs), where do the lone pairs go?
180°
Bonding angle for linear
120°
Bonding angle for trigonal planar
109.5°
Bonding angle for tetrahedral
90° axial → equatorial & 120° equatorial → equatorial
Bonding angle for trigonal bipyramidal
90°
bonding angle for octahedral
Linear
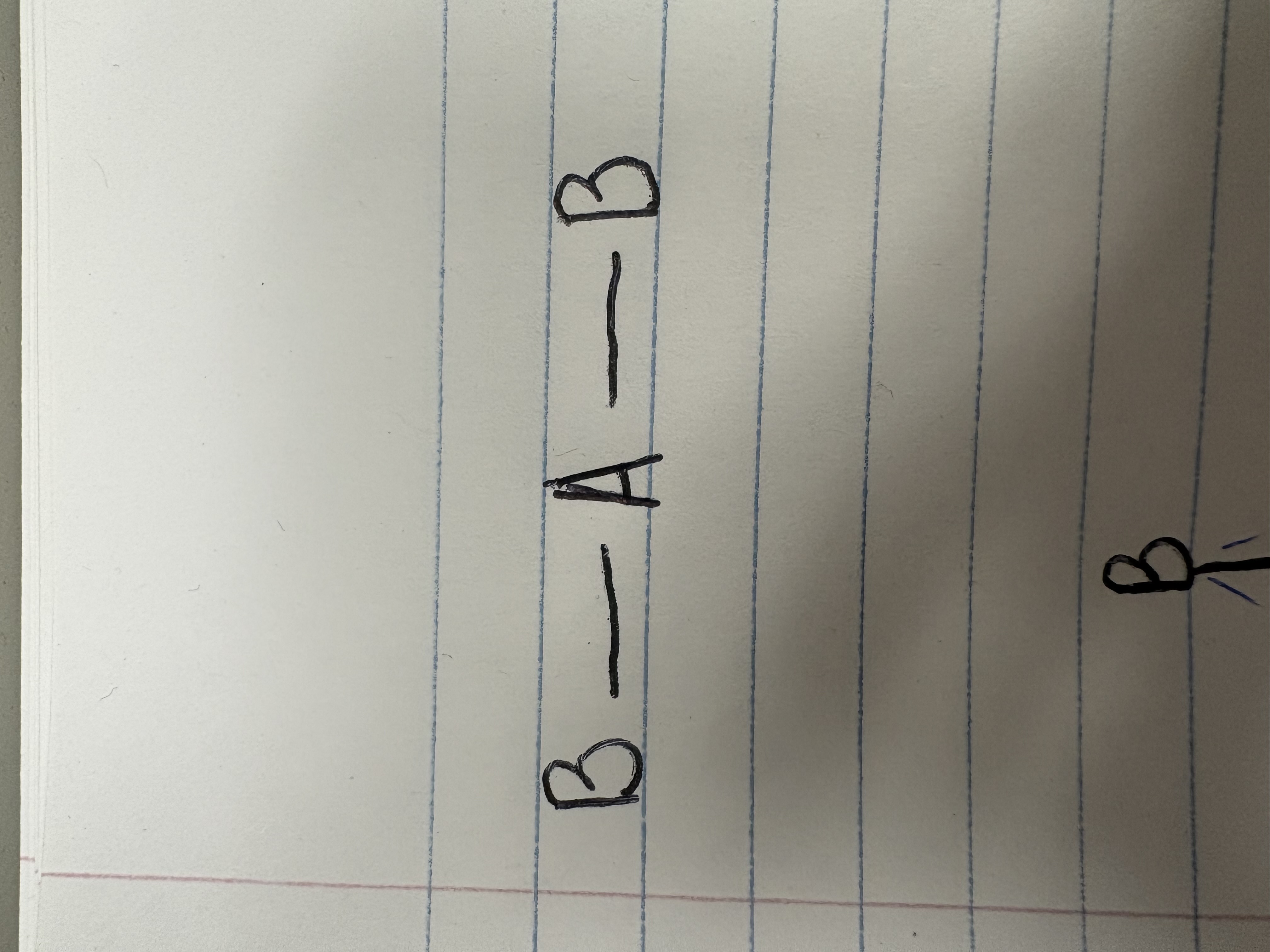
Trigonal planar
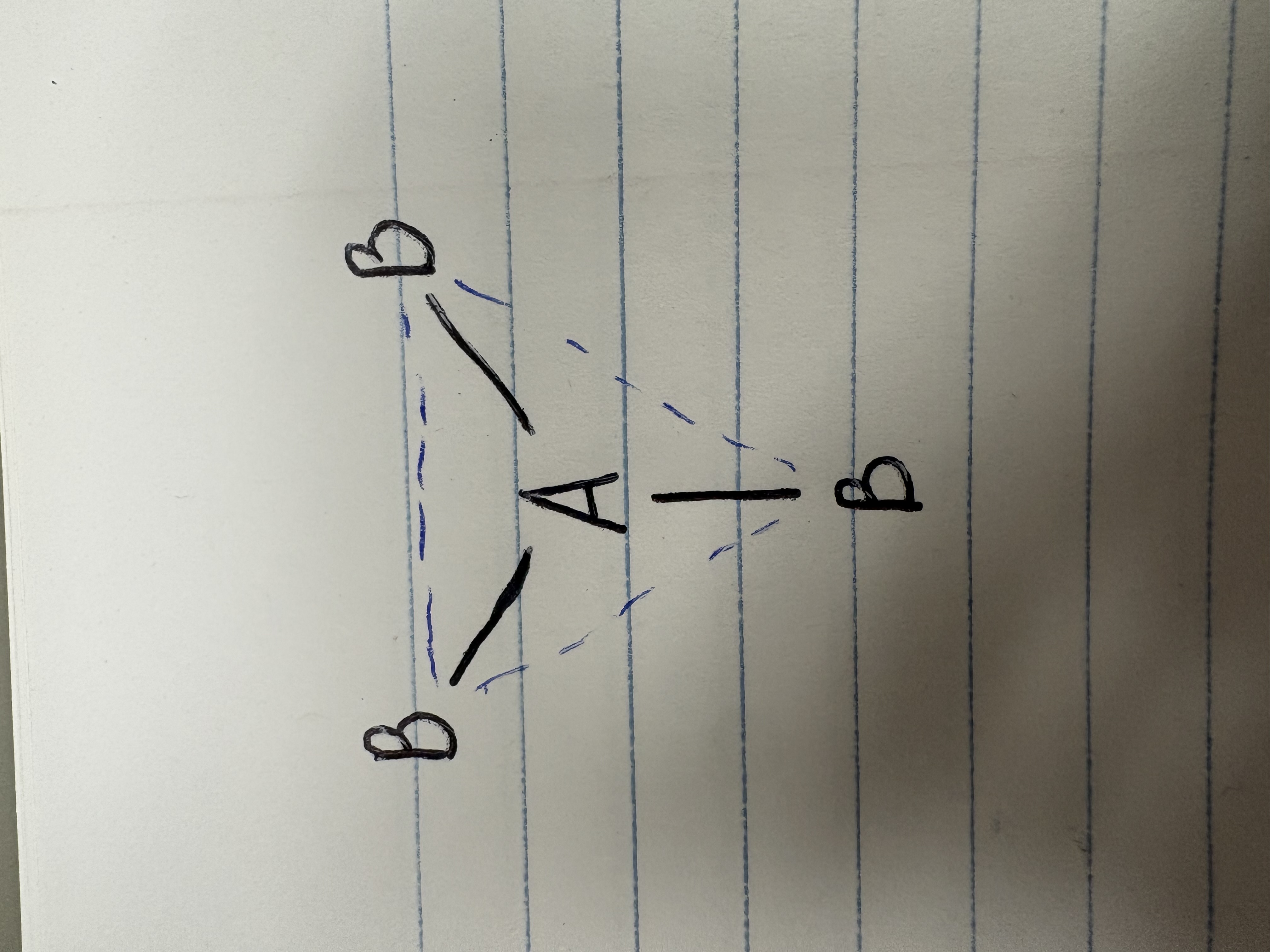
Tetrahedral
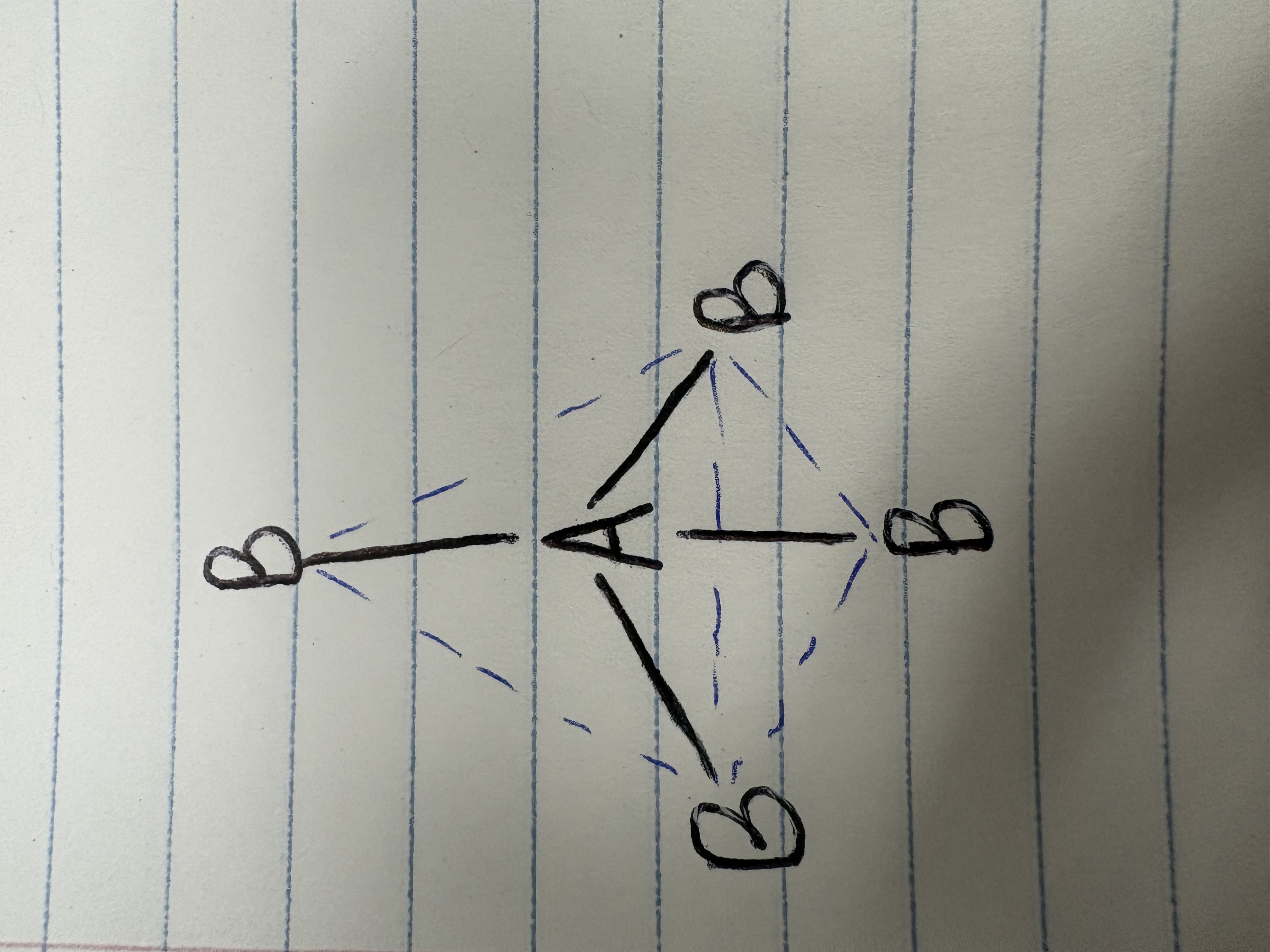
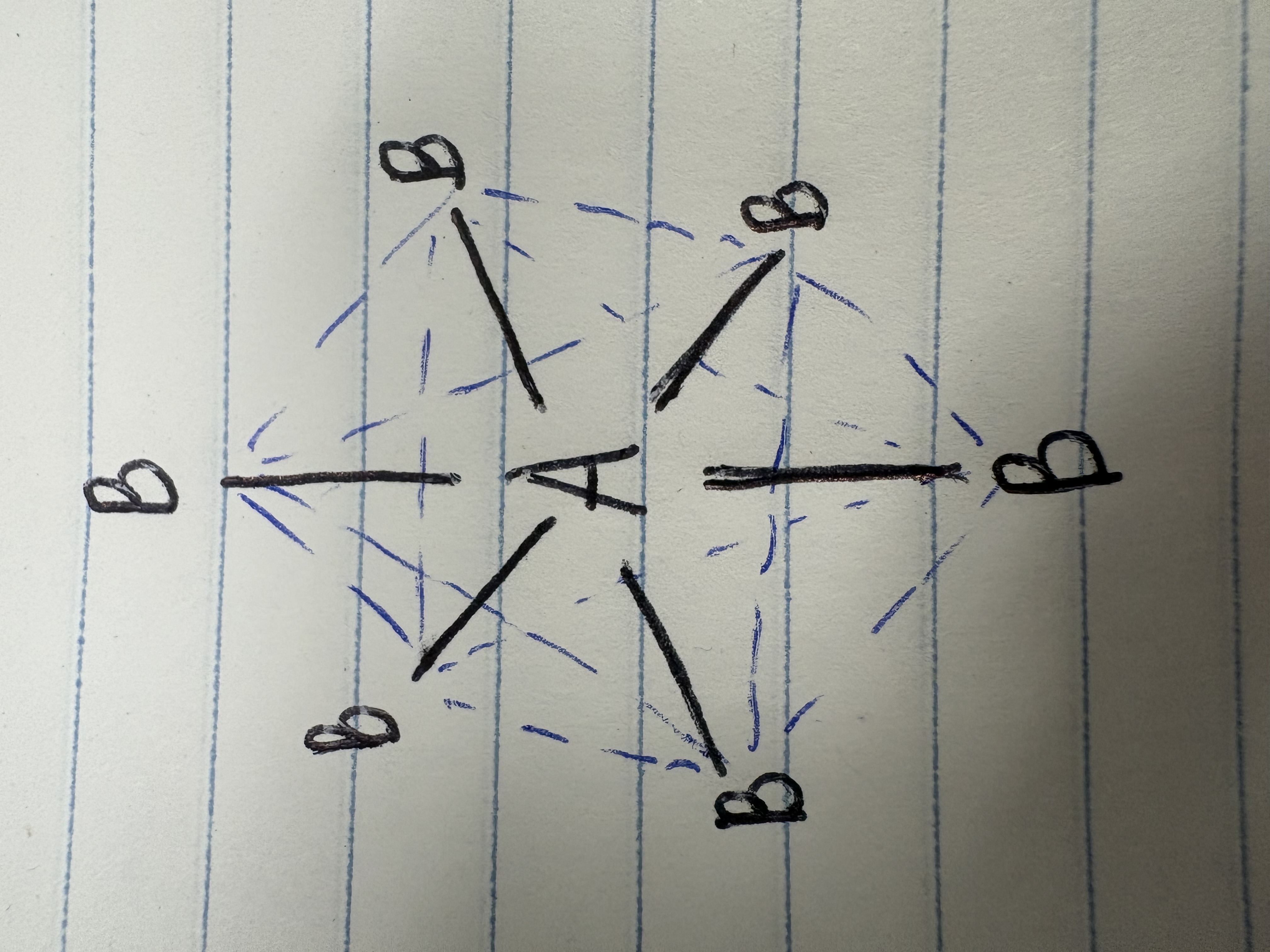
Trigonal bipyramidal
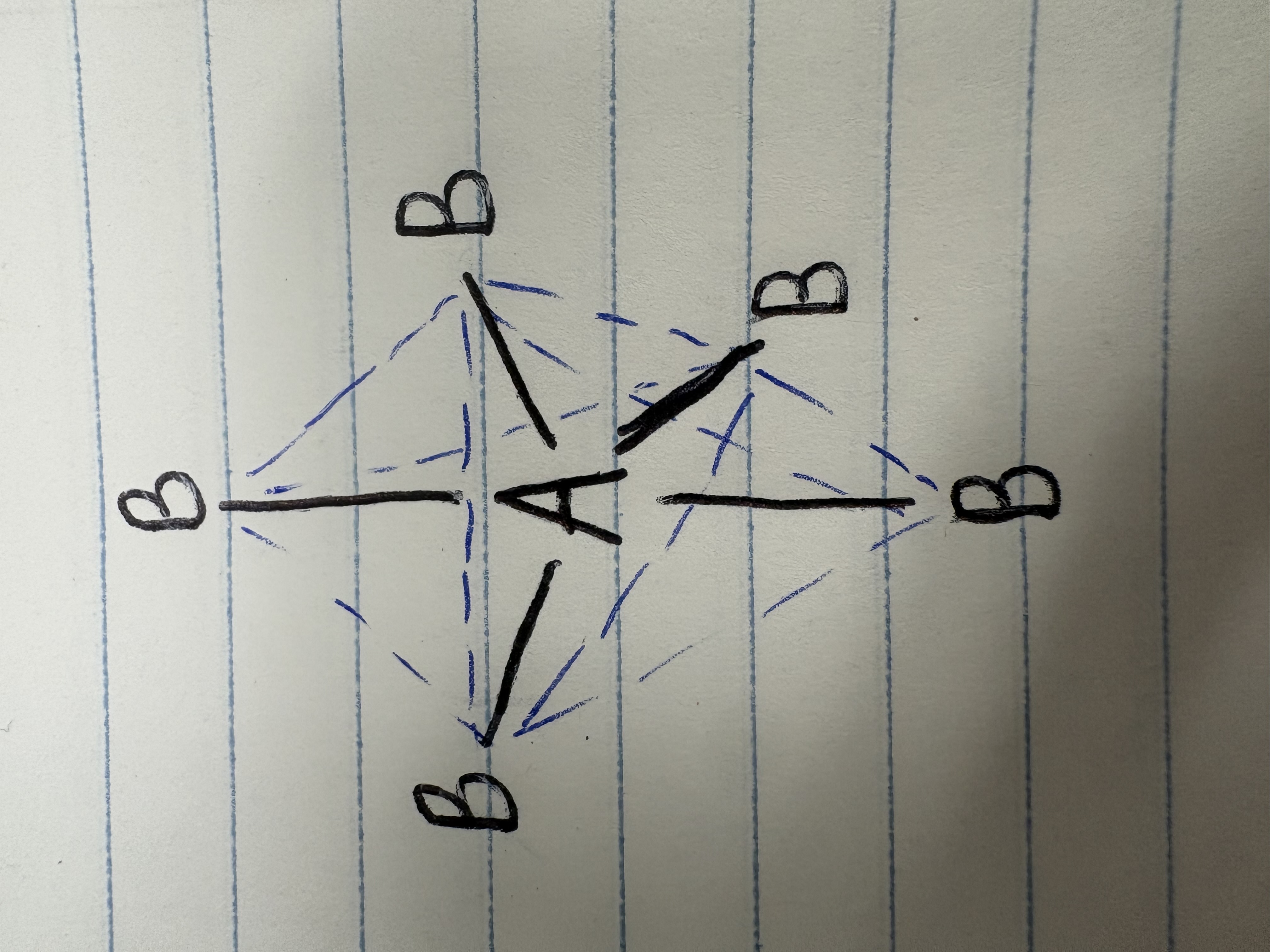
Octahedral
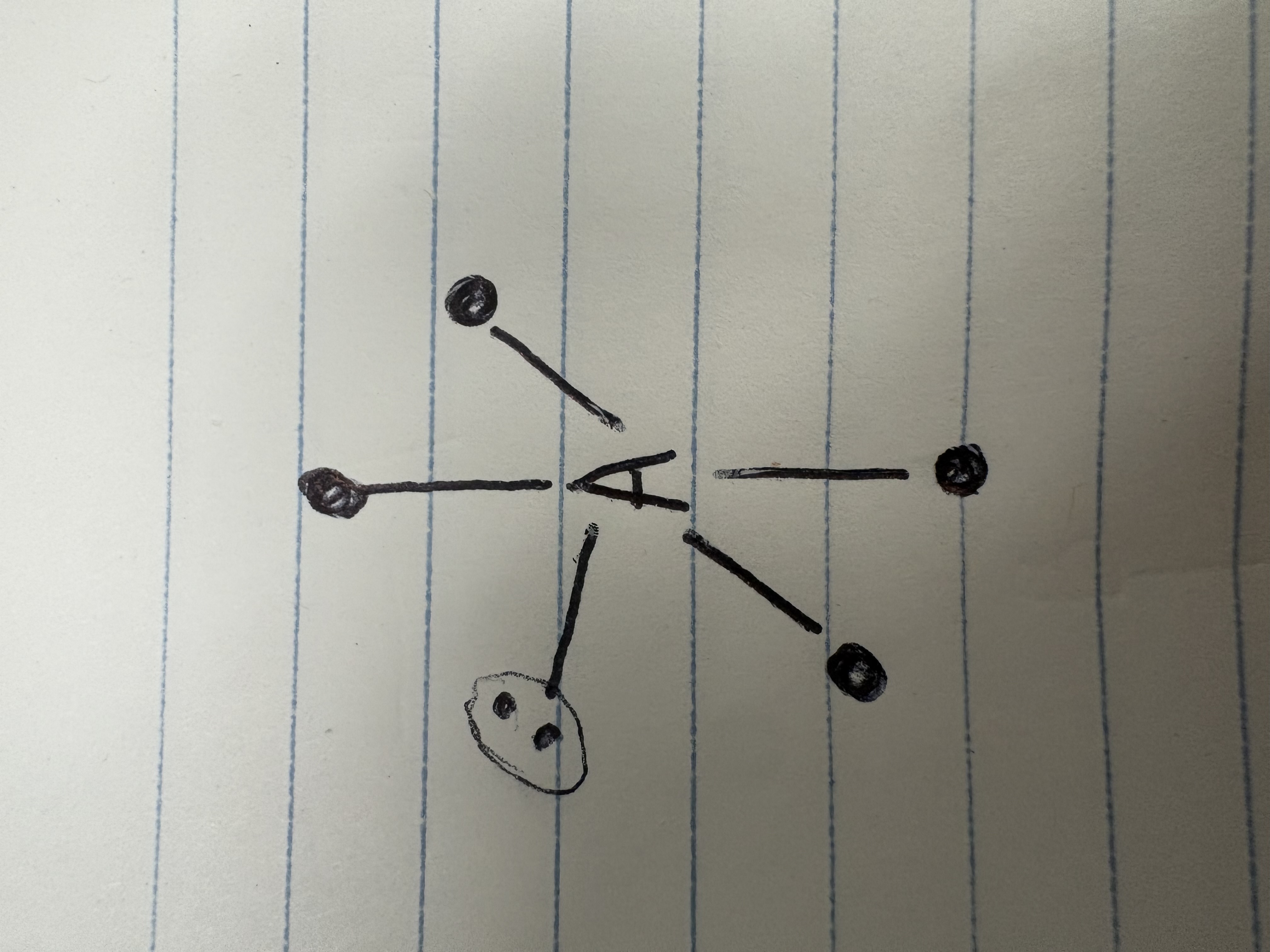
See saw
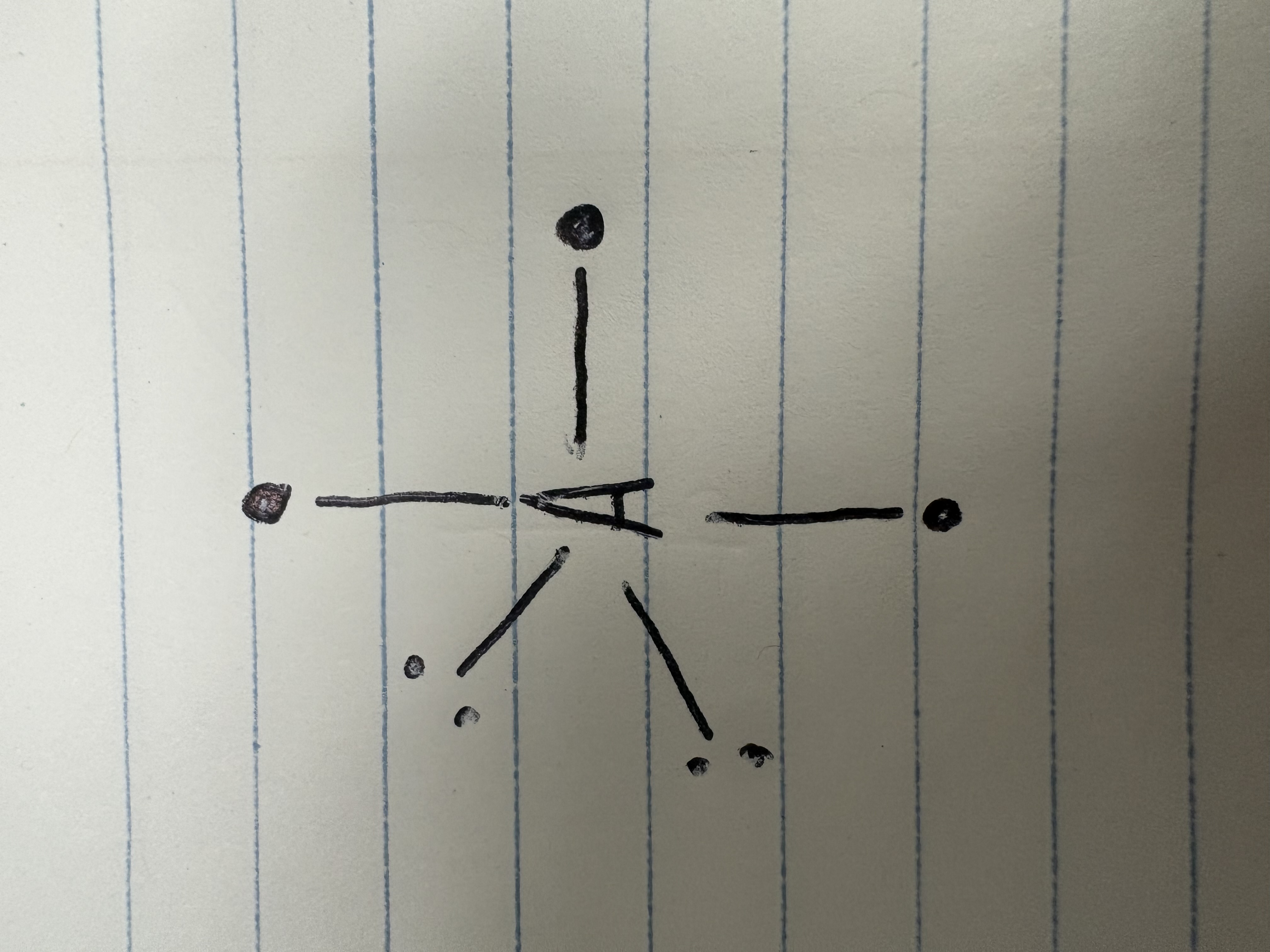
T-shape
Linear (from trigonal bipyramidal)
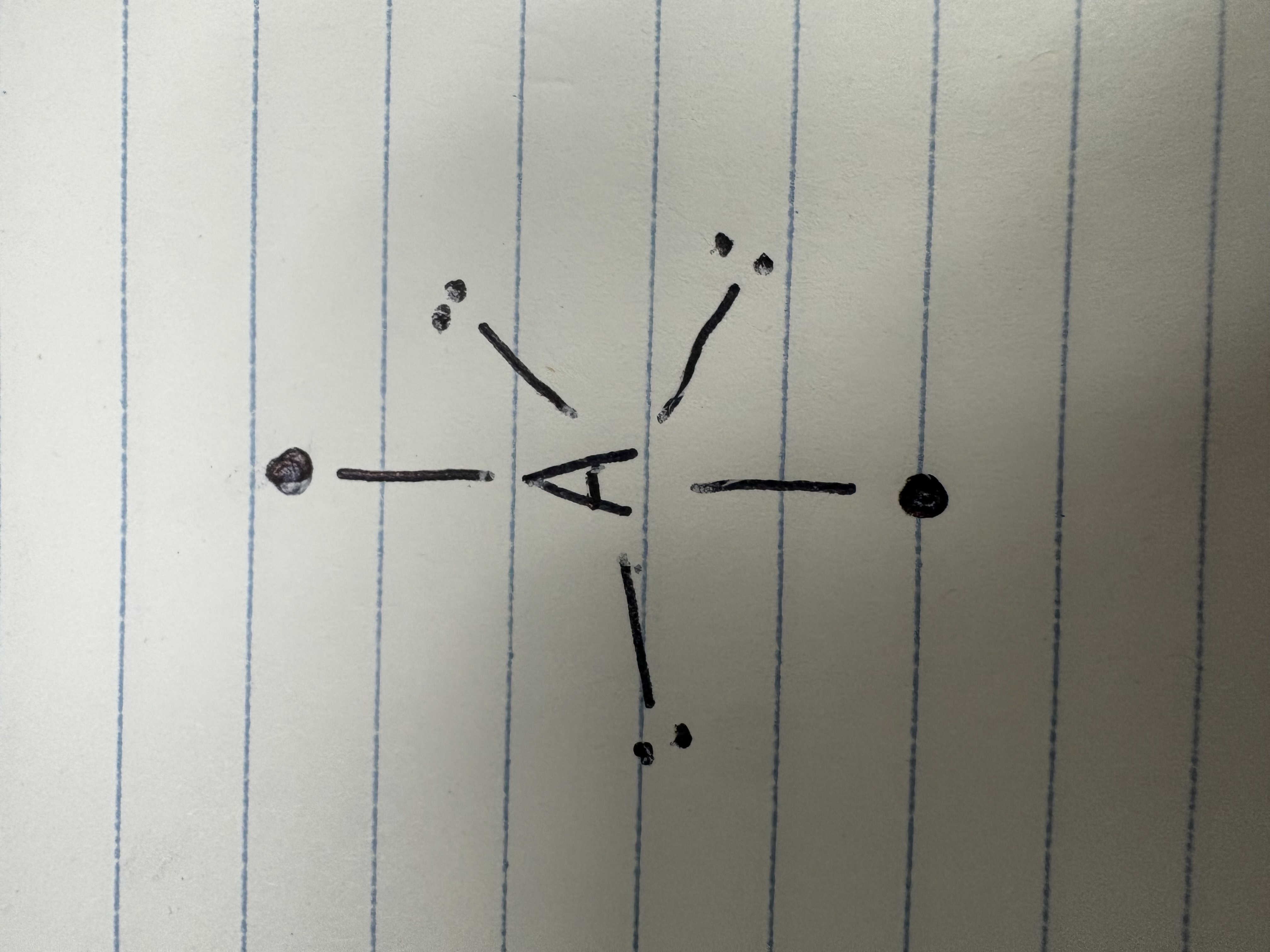
Axial
What direction of bonds is this?
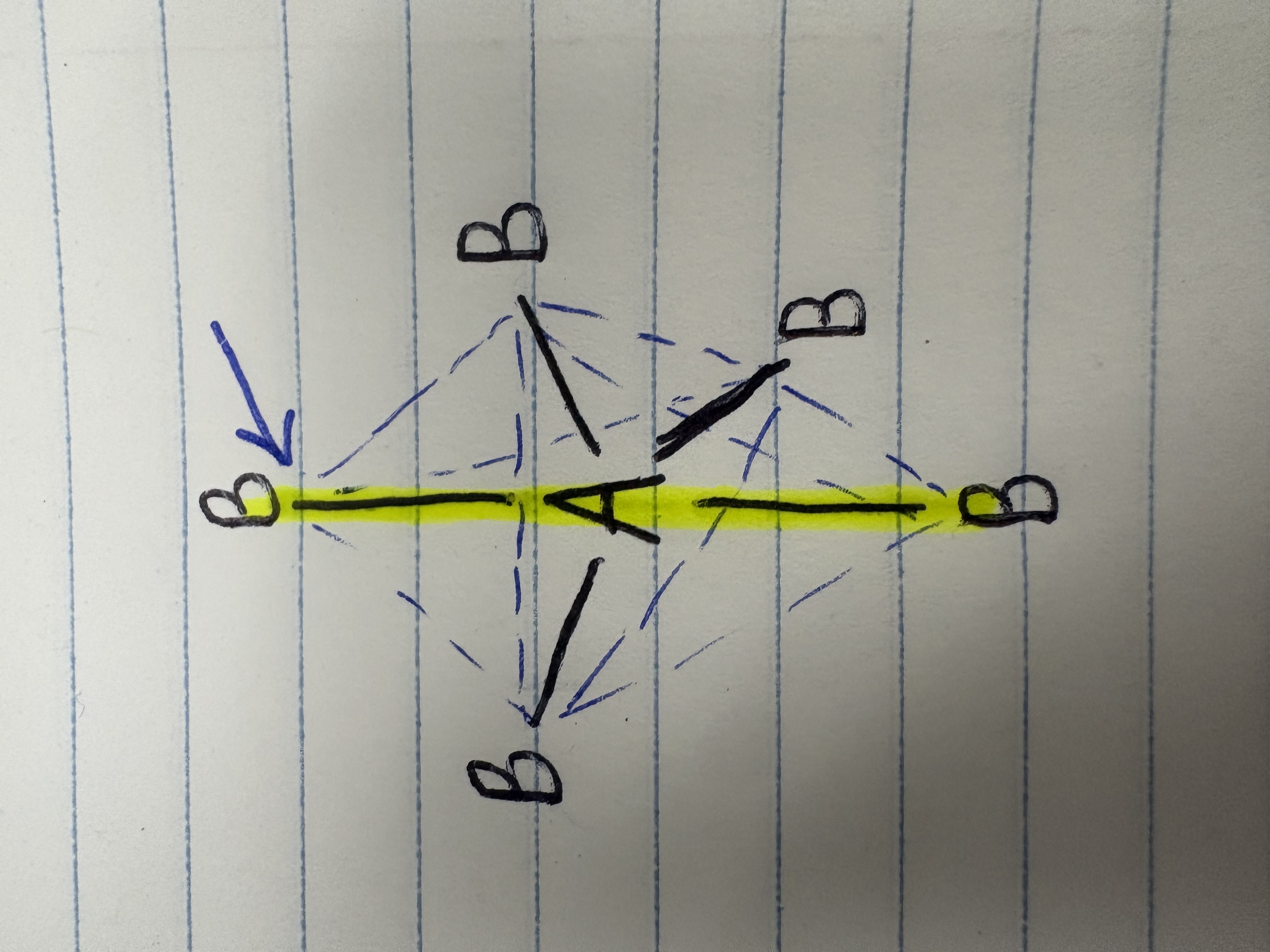
Equatorial
What direction of bonds is this?
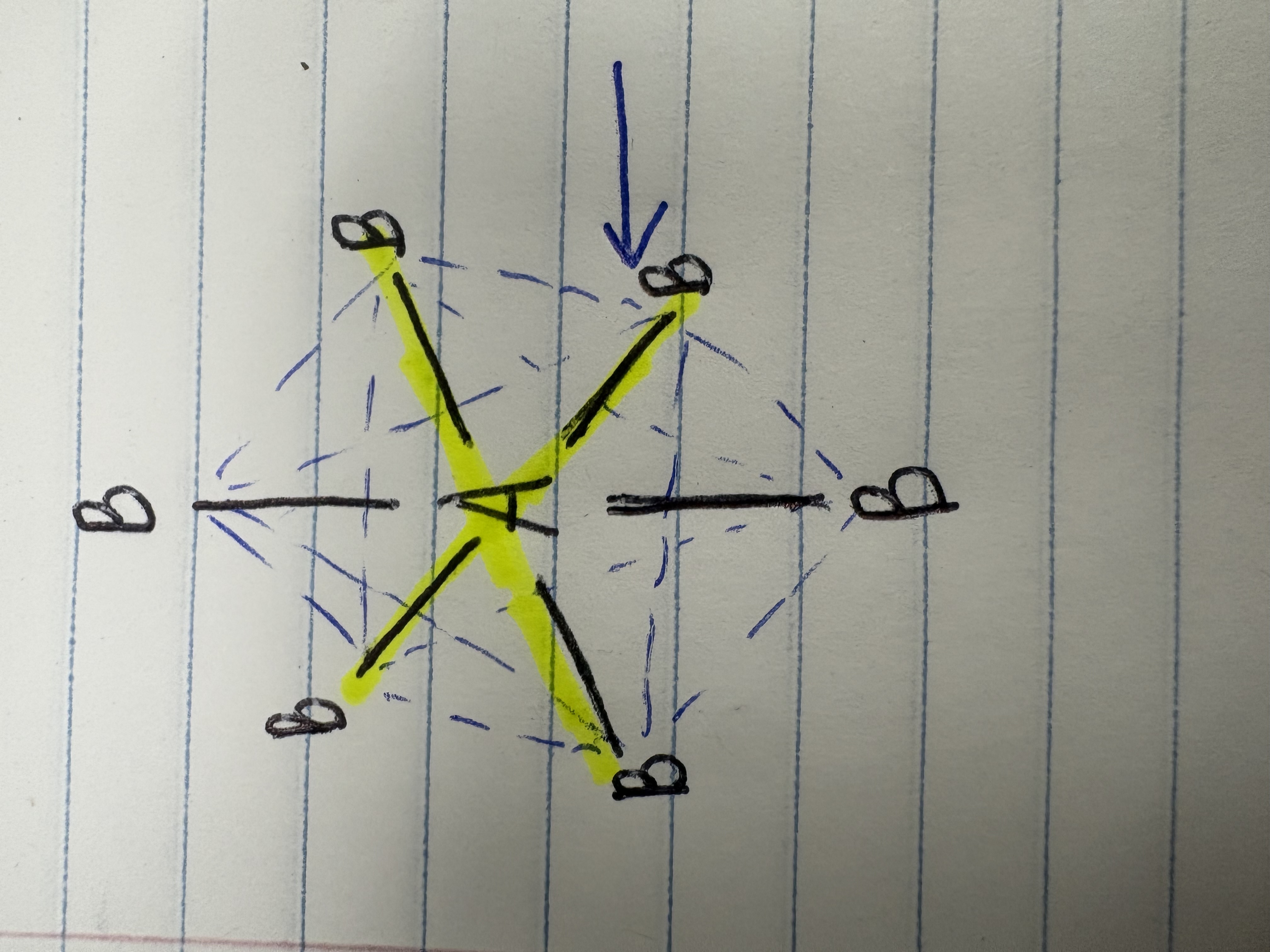
Trigonal pyramidal
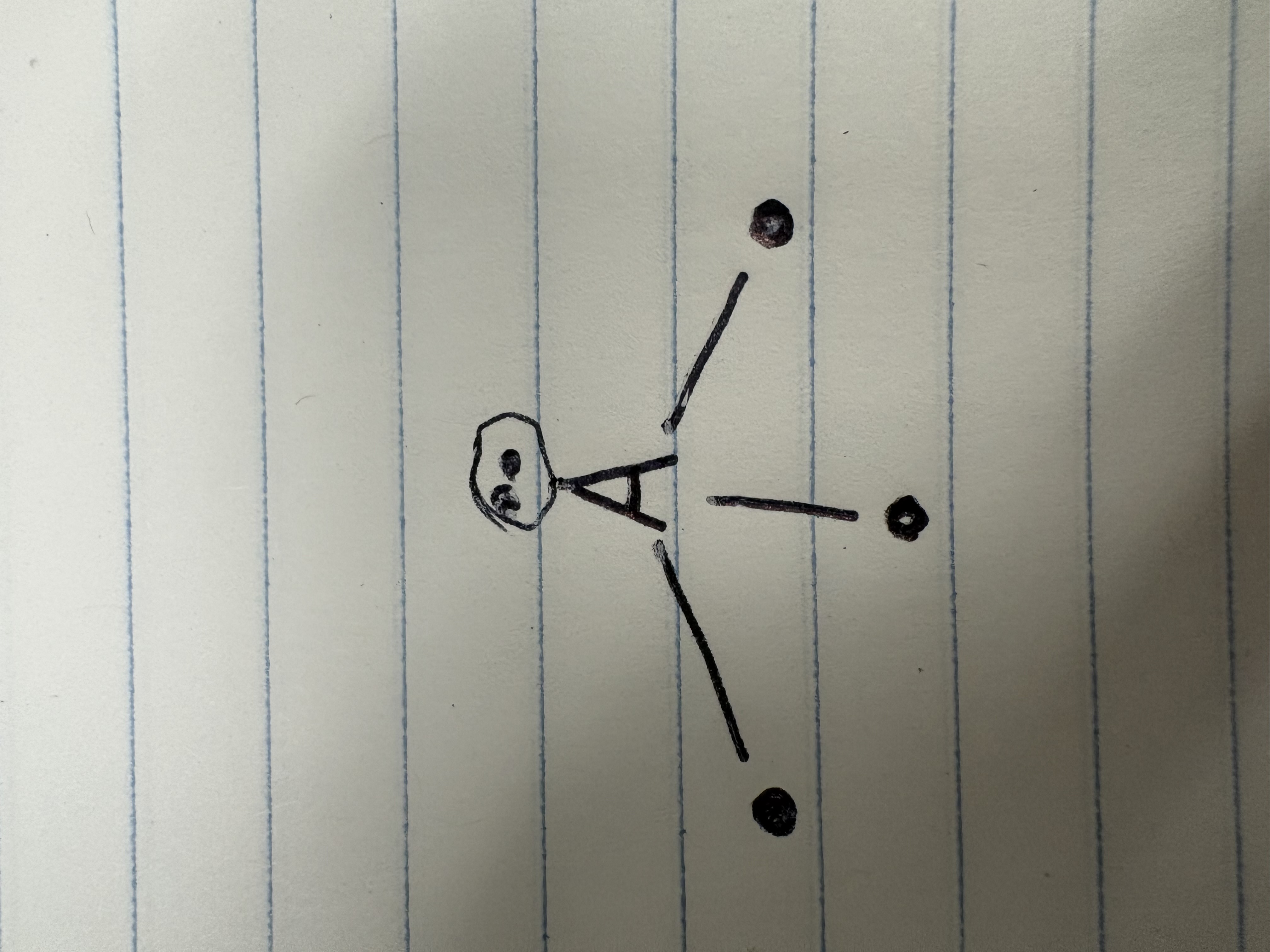
Bent
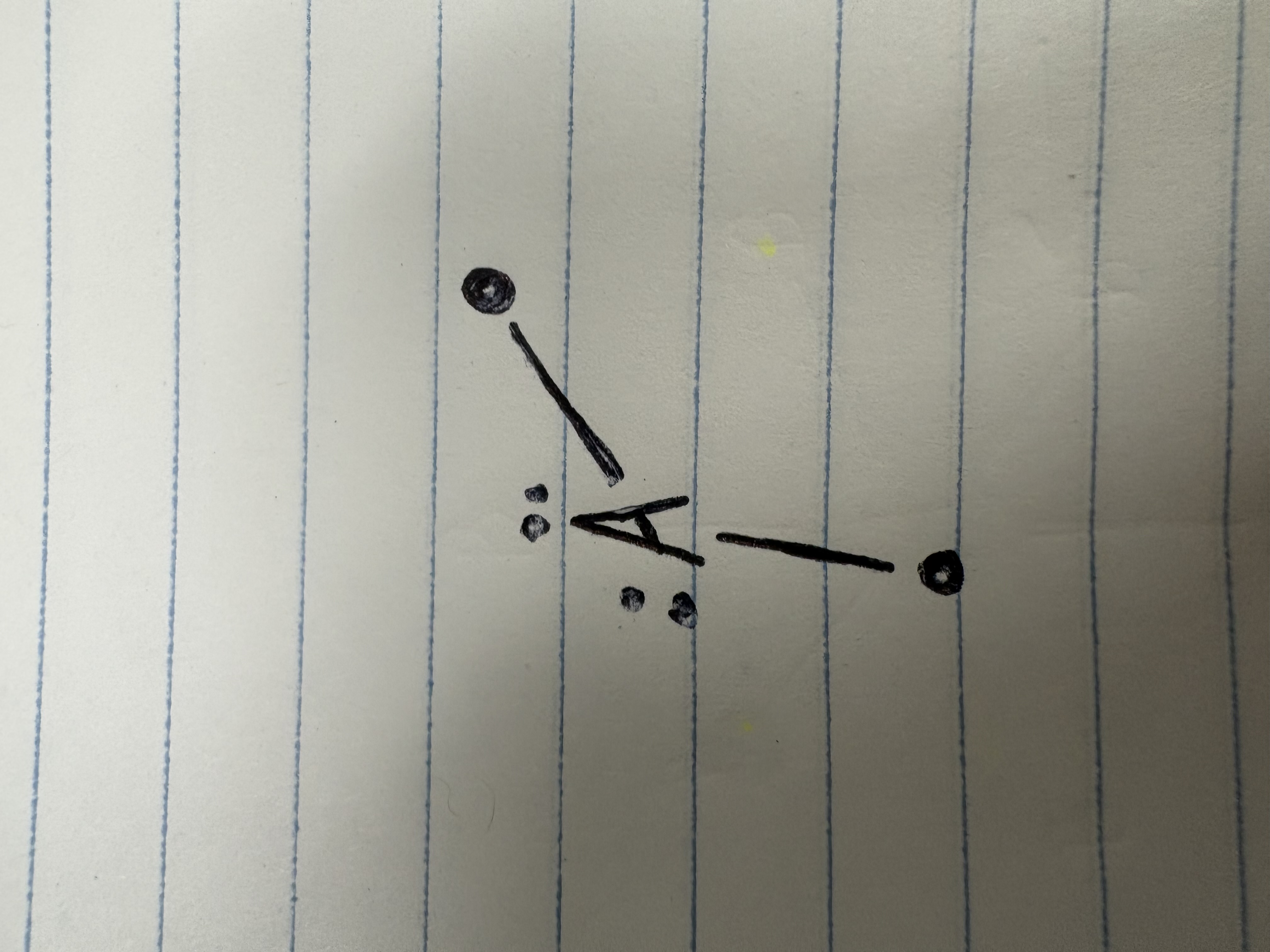
Decrease by 2
when a lone pair replaces a bond in a tetrahedral shape, what happens to bonding angle?
Square pyramidal
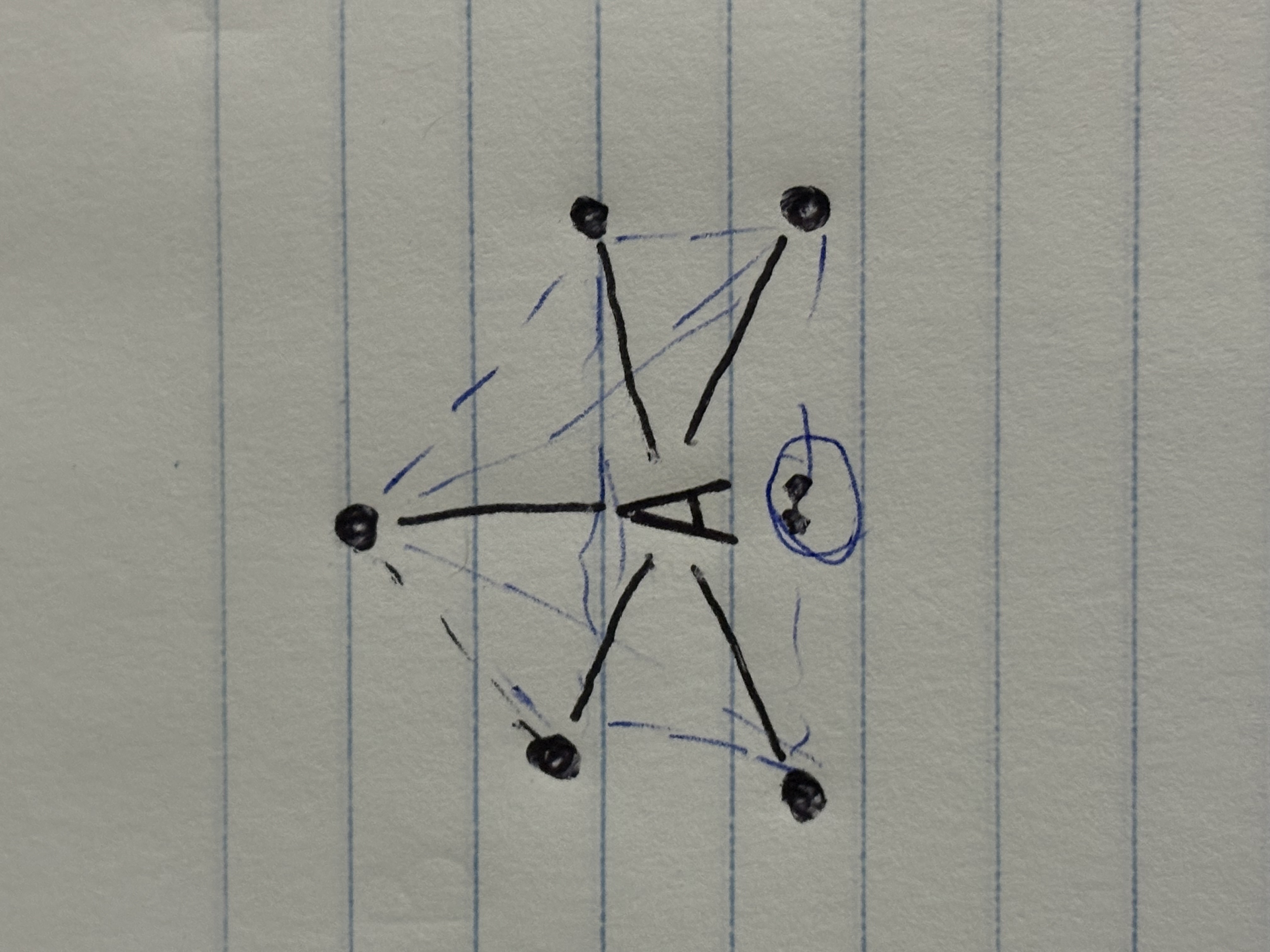
Square planar