Metals and Alloys
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
10 Terms
What do Metals consist of
A giant structure of atoms arranged in regular layers
Describe a metals outer shell
The elections in outer energy level of each atom is delocalised and so are free to move through the whole structure
What is metallic bonding
-Where there are strong forces of electrostatic attraction between the postive metal ions and shared negative electrons. These forces hold the atoms together in a regular structure which is called metallic bonding.These bonds are really strong.
How can bondings in metals be represented
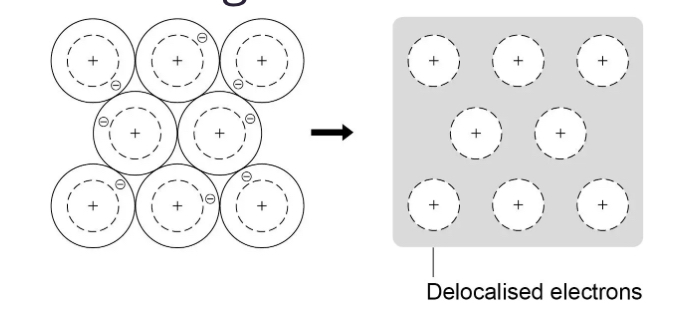
Why do metals have a high melting and boiling point/Solids at room temperature
-The electrostatic forces between the metal atoms and delocalised sea of electrons are very strong so need a lot of energy to break. So metal compound with metallic bonds have a very high melting and boiling point. So they’re generally solid at room temperature
Why are Metals good conductors of heat and electricity
-The delocalised electrons carry electric charge and thermal energy through the whole structure, so metals are good conductors of electricity and heat
Why can metals be bent/shaped
As in metals the layers of atoms can slide over each other making metals malleable
Why can some pure metals not be useful
As they’re often too soft when they’re pure so are mixed with other metals to make them harder
What are alloys
A mixture of two or more metals.
They’re harder and more useful than pure metals
Why are alloys harder than pure metals
As different elements have different sized atoms. So when another element mixed with a pure metal the new atoms will distort the layers making it more difficult for them to slide over each other. Making alloys harder than pure metals