3: Thermal decomposition
0.0(0)
0.0(0)
Card Sorting
1/9
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
10 Terms
1
New cards
decomposition
Chemical reaction in which a compound breaks down to form more than one product
2
New cards
thermal decomposition
Chemical reaction in which a compound breaks down from heating to form more than one product
3
New cards
What is the decomposition reaction of hydrogen peroxide?
* hydrogen peroxide → water + oxygen
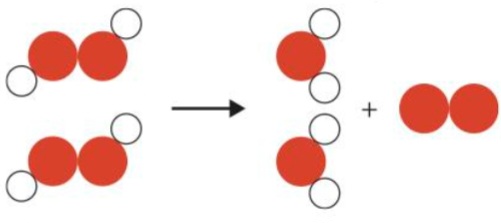
4
New cards
What happens in the thermal decomposition reaction of copper carbonate?
* Copper carbonate is green compound made of copper carbon and oxygen
* When heated, it breaks down to form copper oxide and carbon dioxide
* Copper oxide is black and remains in test tube
* Carbon dioxide is a gas
* When heated, it breaks down to form copper oxide and carbon dioxide
* Copper oxide is black and remains in test tube
* Carbon dioxide is a gas
5
New cards
How do you check if the gas produced is carbon dioxide?
If you bubble it through limewater, and it turns milky, it is CO2
6
New cards
What are type of carbonate that decompose from heating (including the reactants and products)?
* lead carbonate → carbon dioxide + lead oxide
* zinc carbonate → carbon dioxide + zinc oxide
* zinc carbonate → carbon dioxide + zinc oxide
7
New cards
How do you know, from reading a word equation, whether something is a decomosition reaction or not?
The product is simpler than the starting substance and has atoms of smaller numbers and more elements
8
New cards
word equation for decomposition reaction of magnesium nitrate
magnesium nitrate → magnesium oxide + nitrogen dioxide + oxygen
9
New cards
Describe the decomposition reactions of the nitrates of the Group 2 elements as you go down the group.
As you go down Group 2, the elements become more thermally stable and therefore requires more heat to decompose.
10
New cards
State a general rule to predict the products of the thermal decomposition reactions of the Group 2 elements’ nitrates.
The nitrates decompose to make an oxide of the Group 2 metal, as well as nitrogen dioxide and oxygen gases.