4, How the equilibrium constant varies with the difference in energy between reactants and products
1/6
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
7 Terms
Q: What is the equilibrium constant KKK when the energies of reactants and products are the same?
A: When the energies are the same, K=1
Q: How does the equilibrium constant K change when one state has a higher energy than the other?
A: The equilibrium constant KKK becomes larger when the products are much more stable (lower in energy) than the reactants.
Q: What equation relates the equilibrium constant K to the energy difference between reactants and products?
A: The equation is:
ΔG=−RTlnK
Q: What does ΔG represent in the equation ΔG=−RTlnK?
A: ΔG is the free energy difference between the reactants and products, measured in kJ mol⁻¹.
Q: What are the components of the equation ΔG=−RTlnK?
ΔG: Free energy change (kJ mol⁻¹)
R: Gas constant (8.314 J K⁻¹ mol⁻¹)
T: Temperature in kelvin (K)
lnK: Natural logarithm of the equilibrium constant
Q: How does the equation ΔG=−RTlnK help predict equilibrium composition?
A: It allows calculation of the amounts of reactants and products at equilibrium if the energy difference ΔG is known.
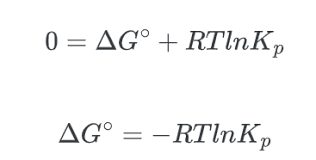
Q: Who derived the relationship between energy and the equilibrium constant?
A: The equation was derived by J. Willard Gibbs, an American physical chemist, in the 1870s.
