Lecture 3 - DVH and Electron Beams
1/130
Earn XP
Description and Tags
ONCOL 356 - Treatment Planning II. University of Alberta
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
131 Terms
What enabled IMRT and what are its main benefits?
Photon beam intensity modulation + inverse planning
improved conformity and better OAR sparing
Why did IMRT increase the need for IGRT?
Higher conformality increased sensitivity to geometric uncertainties
needed more imaging and real-time tracking
What historically limited conventionally fractionated RT?
Normal tissue tolerance (normal tissues were dose-limiting)
Why were large daily doses historically avoided?
Inability to spare nearby critical structures
justification for conventional fractionation
What does SABR/SBRT allow compared to conventional RT?
Large doses per fraction
precise targeting, sharp dose gradients
biologically ablative tumor doses
How have technological advances changed radiotherapy indications?
Improved tumor control with acceptable toxicity, thus expanded indications for RT
What is QUANTEC (2010)?
Evidence-based summary of dose–volume effects on normal tissues (Quantitative Analyses of Normal Tissue Effects in the Clinic)
How is QUANTEC used clinically today?
Provides practical dose constraints mainly for conventionally fractionated RT (e.g., max point dose + volume dose parameters as safety checks)
What are the key limitations of QUANTEC?
Pooled heterogeneous data; constraints only valid where sufficient clinical data exist
How is SABR conceptually similar to surgery?
Both accept predictable normal tissue injury to maximize tumor eradication; risks are weighed via informed consent
What defines radiotherapy ablation?
Doses sufficient to stop proliferation and disrupt tissue function, increasing tumor control toward surgical outcomes
Why can’t SABR toxicity be extrapolated from conventional fractionation models?
Ablative doses cause focal, potentially severe injury unlike low-dose fractionated effects
What factors determine acceptable normal tissue risk in ablative RT?
Severity of harm, patient factors, treatment intent, disease aggressiveness, and timing of injury
What are the two central questions in ablative radiotherapy?
will the dose cure the tumor?
what is the extent/reversibility of normal tissue injury?
What is the difference between structurally defined and undefined FSUs?
Defined: discrete anatomical units, limited clonogen migration (e.g., lung alveoli)
Undefined: continuous structure, free clonogen migration (e.g., esophagus)
What defines parallel functioning tissues?
Independent, redundant FSUs; function depends on volume spared (e.g., lung, liver, kidney, breast)
What defines serial functioning tissues?
Chain-like function; damage to one segment can disable the organ (e.g., spinal cord, GI tract, bowel)
How does radiation injury differ between parallel and serial tissues?
Parallel: volume-dependent toxicity (Vx, V20); number of FSUs damaged matters
Serial: dose-dependent toxicity; maximum (critical) dose is key
Why is technology more beneficial for parallel tissues than serial tissues?
It reduces irradiated normal tissue volume but cannot protect serial organs adjacent to high-dose targets
How does tissue regeneration affect tolerance to ablative doses?
Regenerative capacity improves tolerance, but requires preserved clonogens, vasculature, and stroma
Why is the Linear Quadratic (LQ) model limited for SABR?
It’s derived from low-dose data, overestimates toxicity at ablative doses, and doesn’t fit high-dose survival behavior
What is the Universal Survival Curve (USC)?
A hybrid model using LQ at low doses and a multitarget asymptote at high doses, better fitting ablative-dose data
What are the main limitations of current SABR toxicity data?
Mostly retrospective data with nonuniform delivery, inconsistent reporting, selection bias, and variable dose constraints
How may advanced imaging improve SABR toxicity assessment?
MRI and virtual endoscopy may better detect and predict radiation-induced tissue damage
What is the ultimate goal of ongoing SABR research?
Maximize cure while minimizing normal tissue toxicity
How have external beam RT techniques evolved since the late 1990s?
3DCRT has largely been replaced by IMRT and advanced techniques like VMAT, Tomotherapy, and CyberKnife
What planning and computing advances have impacted modern RT?
Faster computers, improved graphics, easier IMRT/VMAT planning, and routine PET/MRI registration with planning CT
What defines SBRT/SABR and why is it significant?
High-dose RT in 1–10 fractions enabled by IGRT; a major paradigm shift with rapidly expanding use
What clinical outcomes are associated with SBRT?
High tumor control, low serious toxicity, and some novel toxicities with unclear mechanisms
Why does SBRT generally have a favorable toxicity profile?
Very accurate setup and treatment of small tumor volumes (<100 cc)
What is adaptive radiotherapy and why is it important?
lan modification during treatment to account for anatomical changes, enabled by IGRT and fast planning
What is the role of QUANTEC in modern RT?
Provides dose–volume guidelines, summarizes toxicity data, and identifies areas needing further research
What advantages do modern planning systems offer over traditional 3-plane review?
Fast volumetric dose calculation, full-volume scrolling, and easy detection of hot/cold spots
How can unintended high-dose regions be reduced during planning?
Modify beam number/directions, use higher-energy beams, or apply dose-control (dummy) structures
What is the difference between differential and integral (cumulative) DVHs?
Differential shows volume per dose bin; integral shows volume receiving ≥ a given dose
What are the main clinical uses of differential vs integral DVHs?
Differential → assess dose range/uniformity and hot/cold spots
Integral → primary tool for clinical evaluation
What is the key limitation of DVHs?
They contain no spatial/anatomical dose information and must be paired with isodose review
What defines a “good” target DVH in conventional RT?
Most of target receives prescription dose (e.g., D95 ≈ Rx) with min/max ~90–110%
Which DVH metrics are most important for serial vs parallel organs?
Serial → maximum dose (Dmax)
Parallel → volume-based metrics and mean dose
Why is mean organ dose clinically important?
Strongly correlates with toxicity (lung, parotid, liver, kidney)
What is NTCP modeling and how is it used clinically?
Normal tissue complication probability
Uses entire DVH to estimate complication risk
What are the competing goals in RT planning?
Balancing target coverage with normal tissue protection while remaining safe, effective, and feasible
What are non-negotiable planning constraints?
OAR limits that must be met even if target coverage is compromised (e.g., spinal cord Dmax < 50 Gy in conventional RT)
What is knowledge-based planning (KBP)?
Use of prior high-quality plans to predict achievable OAR DVHs based on patient anatomy
What are the main benefits and limitations of KBP?
benefits: reduced planning time, more consistent plan quality and more objective plan evaluation
limitation: extensive comissioning and limited transferability between institutions
What are the future implications of KBP?
Improved plan quality, fewer complications, faster planning, and evolving plan quality metrics based on outcomes
What currently guides most clinical RT planning and evaluation?
Dose–volume criteria; biologic models exist but are not yet routine
What treatment paradigm is currently driving change in RT planning?
Hypofractionation
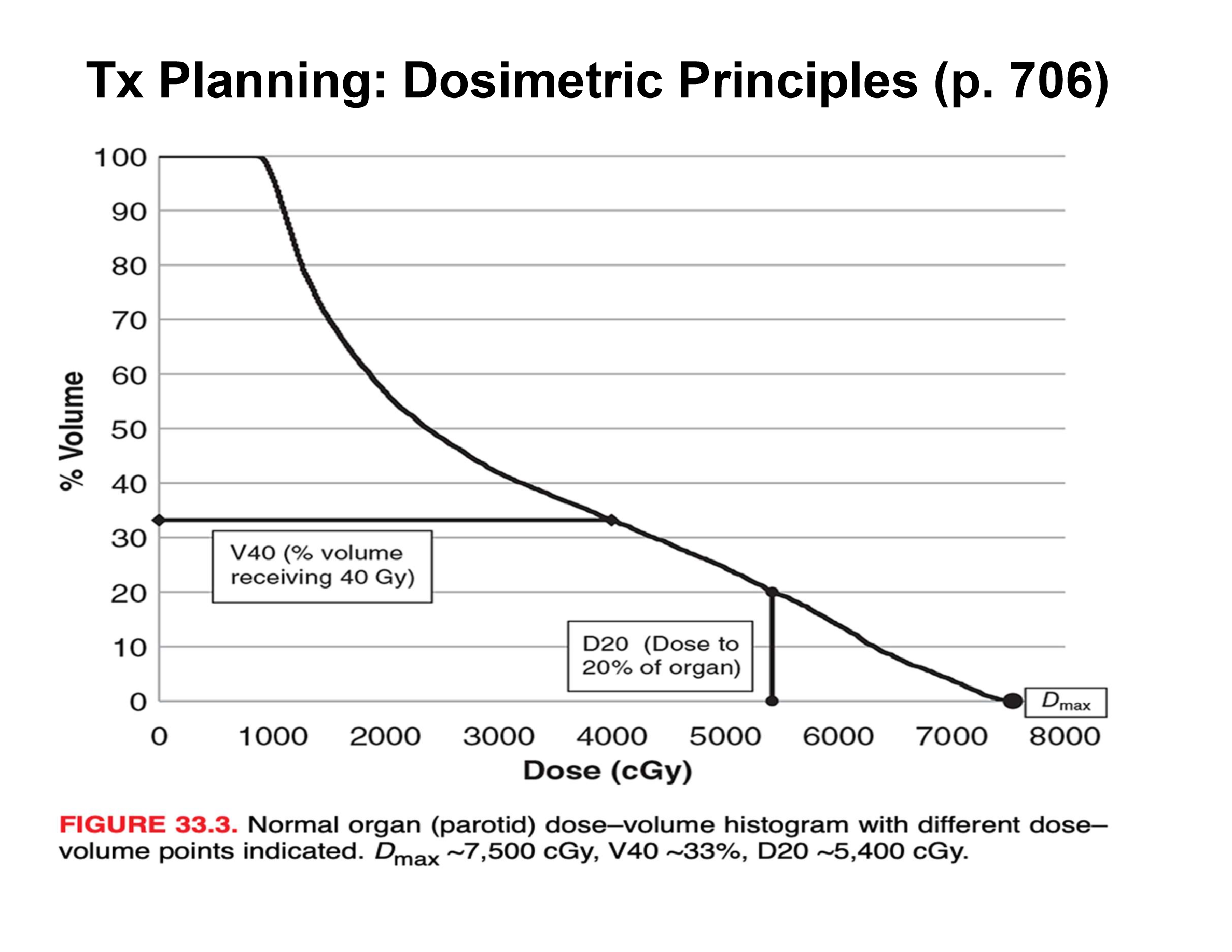
what is the V40 and D20?
V40 = percent volume receiving 40 Gy = 33%
D20 = dose to 20% of organ = 54 Gy
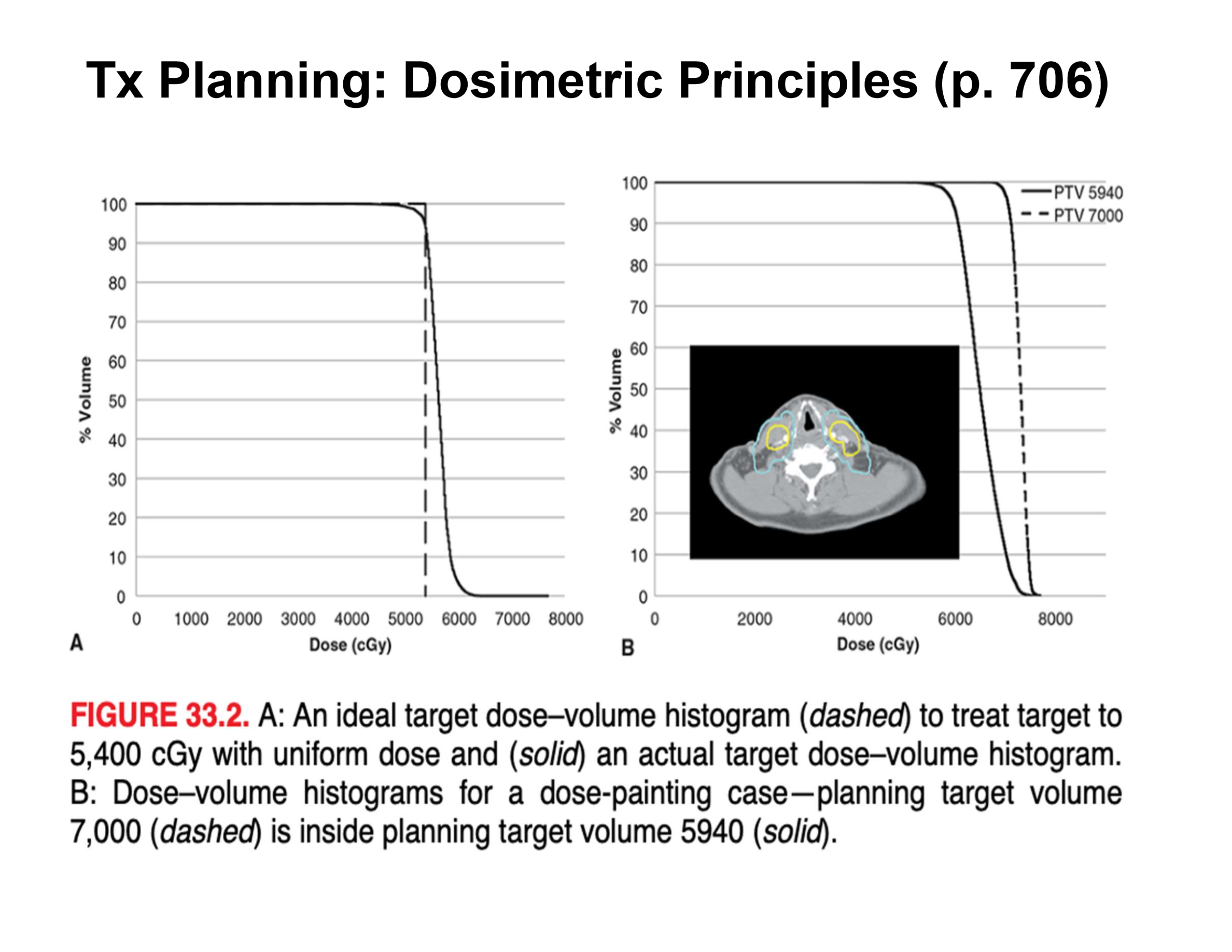
what do the dotted and dark lines tell us
for the DVH, ideally a perfect line would be the dotted one where the shoulder drops immediatley for dose to an organ but we get more of a ‘gradual’ drop-off
what are the hotspot values for: 3D-CRT, IMRT, SABR
3D-CRT = keep below 110%
IMRT = below 110%, usually lower than 3D-CRT
SABR = we want hotspots in tumor → 130%
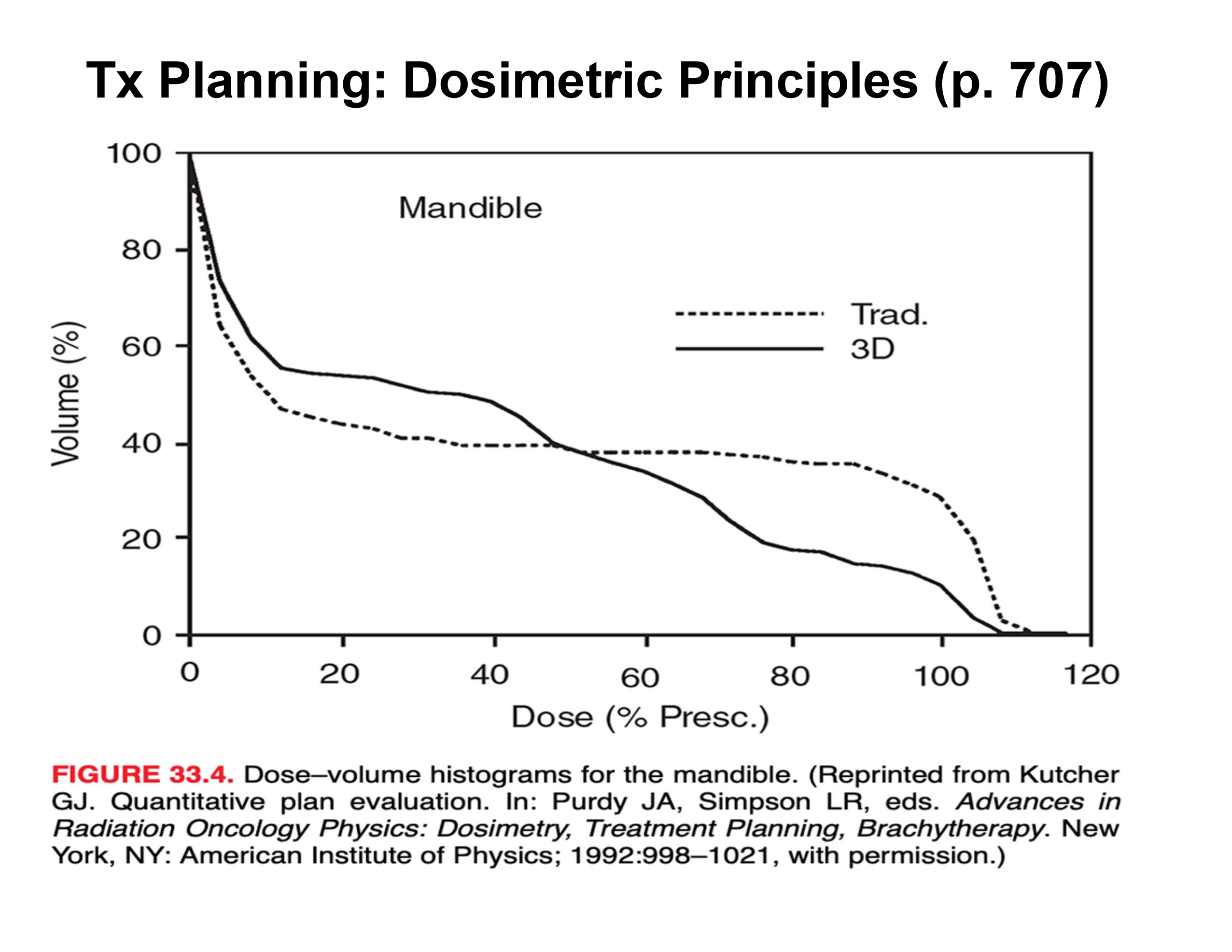
what is this graph telling us
with IMRT and VMAT we are getting better dose coverage and drop-off than just traditional models
what do clinical trials look at?
risks and causes (genetics, lifestyle, etc increase cancer risk)
preventing cancer (using drugs or lifestyle risks)
screening (tests for people with higher risk of cancer)
diagnosing cancer (new scans, tests, procedures)
treatments (new drugs / combo drugs)
controlling symptoms or side effects (new drugs or therapies)
support and information
what are the two types of trials?
interventional studies and observational studies
interventional studies
aim to find out more about a particular intervention or treatment
observational studies
aim to find out wht happens when people are put in different situations
observe people and don’t influence what treatments people have
examples of trials within interventional and observational
pilot studies and feasibility studies, prevention trials, screening trials, treatment, multi-arm multi-stage (MAMS), cohort, case control, cross sectional
what are trial phases?
clinical trials testing new treatments are divided into different phases
the earliest phase may look at whether a drug is safe or side effects it causes
later phase trials aim to see if new treatment is better than existing ones
what are the three main phases of clinical trials
phase 1-3
some have phase 0 and 4
phase 1 trials

phase 2 trials

phase 3 trials

phase 4 trials

What is the typical clinical energy range of electron beams and their effective treatment depth?
~6–20 MeV; effective for tumors up to ~6 cm depth
What are common clinical applications of electron therapy?
Skin cancers, chest wall irradiation, boost treatments (breast, head & neck), and IORT
What are the main advantages of electrons compared to X-rays?
Higher surface dose, finite range with minimal exit dose, and better sparing of deeper tissues
How have IMRT and IGRT reduced reliance on electron therapy?
IMRT can achieve similar dose distributions without electron field complexity, and IGRT improves accuracy
How do electrons primarily lose energy in tissue?
By collisions with orbital electrons; ~2 MeV per cm of water in low-Z tissue
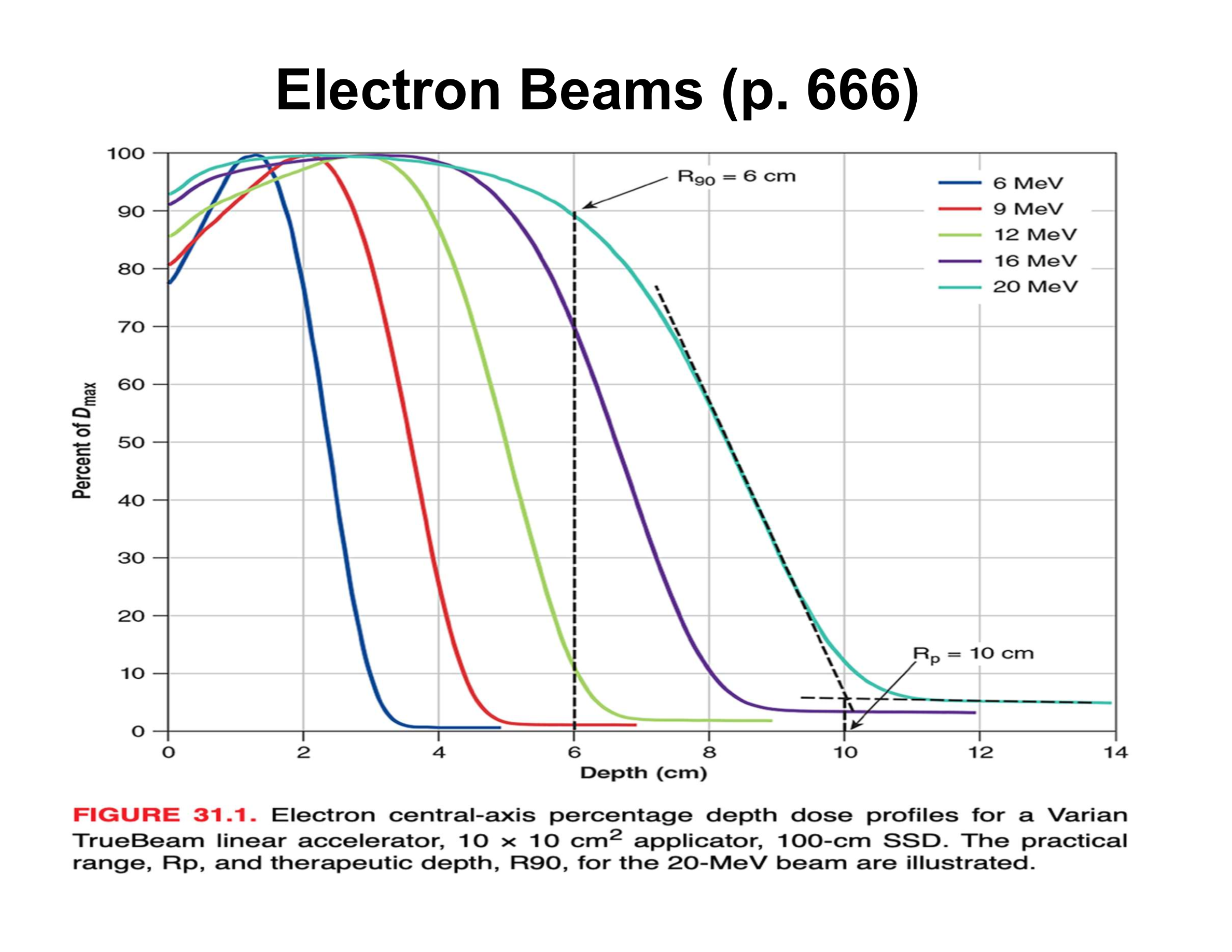
What is the practical range (Rp) in an electron PDD?
Depth where the distal falloff intersects the bremsstrahlung tail; beyond Rp dose is mainly X-ray contamination
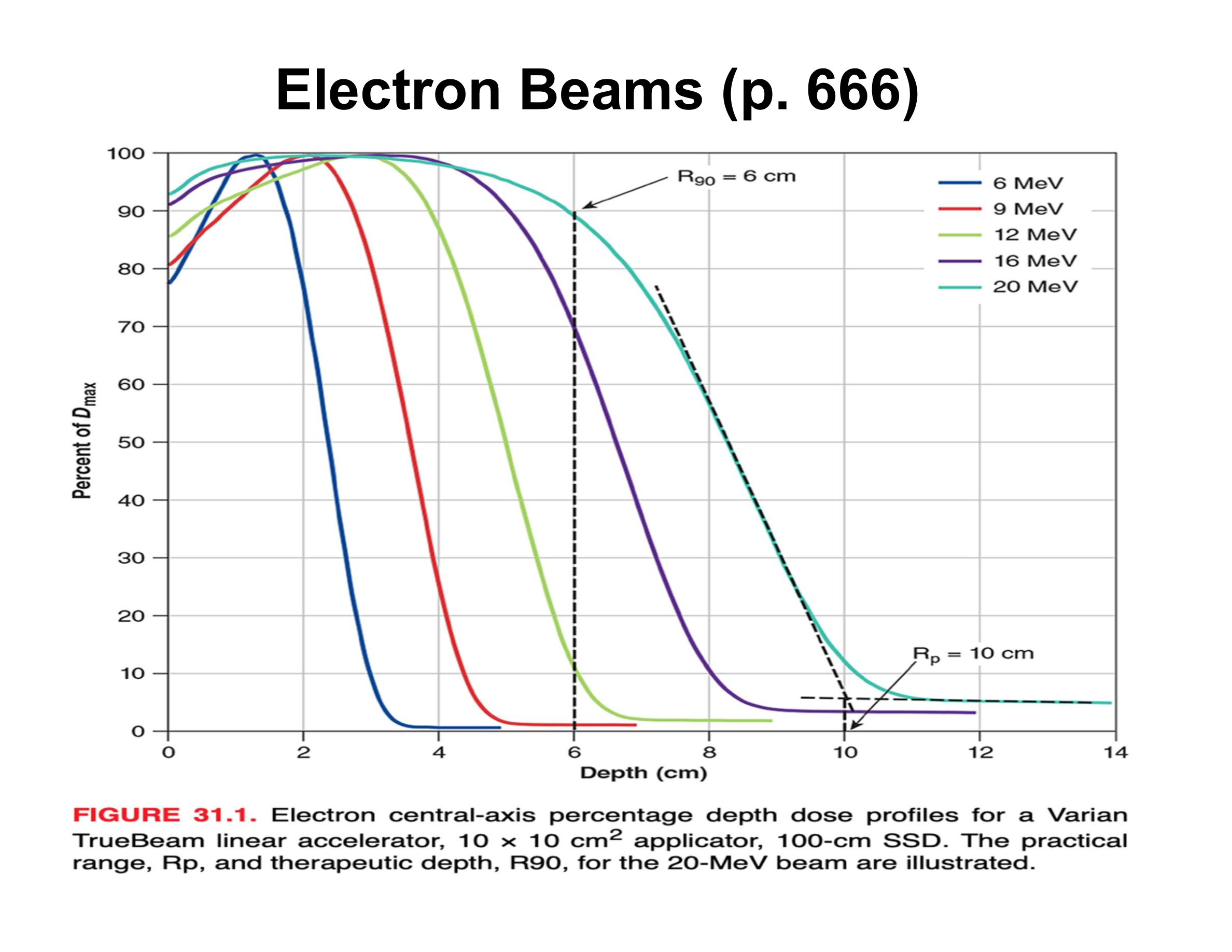
What causes the sharp distal falloff in electron beams?
Multiple Coulomb scattering; very few electrons actually reach Rp
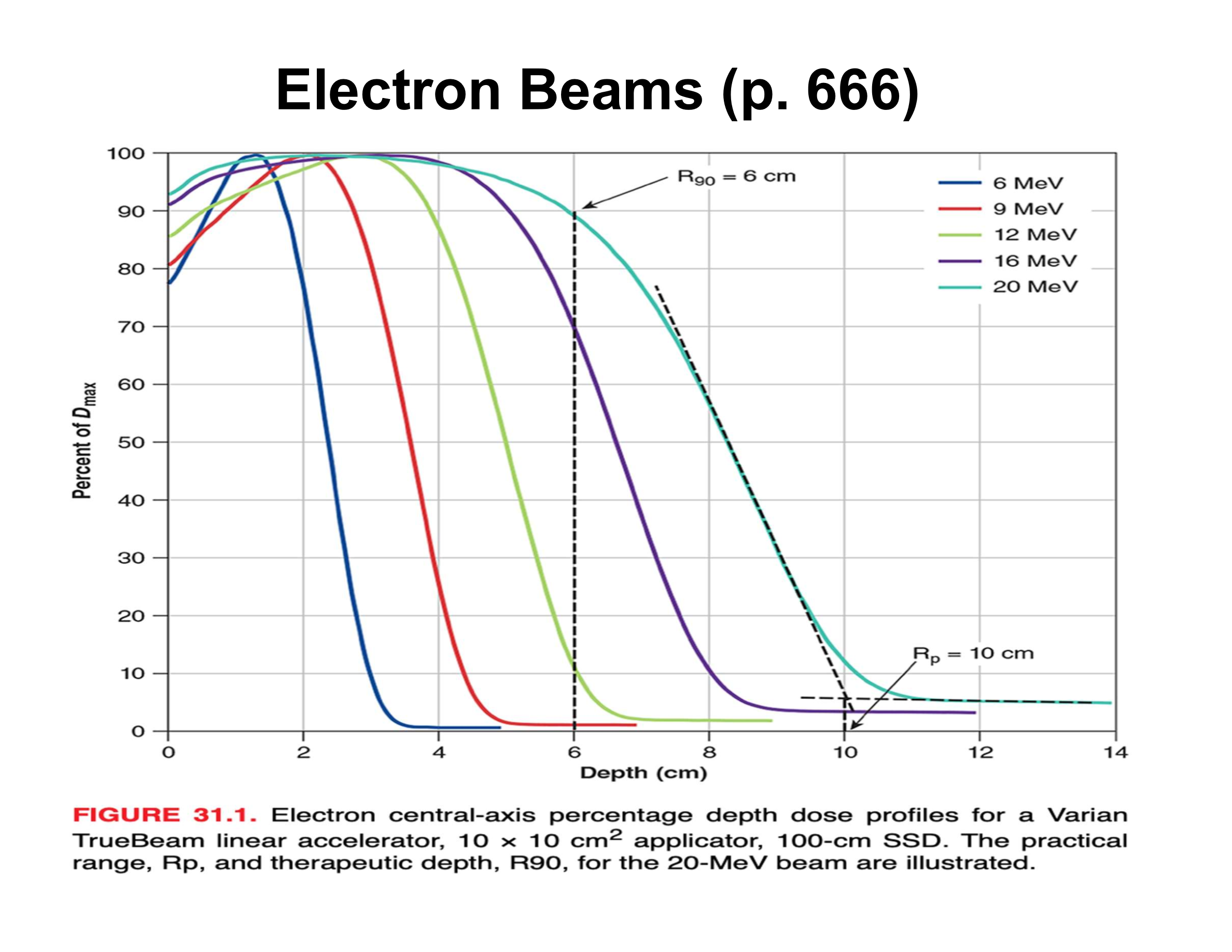
What is the relationship between electron beam energy and practical range?
Beam energy (MeV) ≈ 2 × Rp (cm)
How is therapeutic depth related to electron energy?
Increases almost linearly with energy
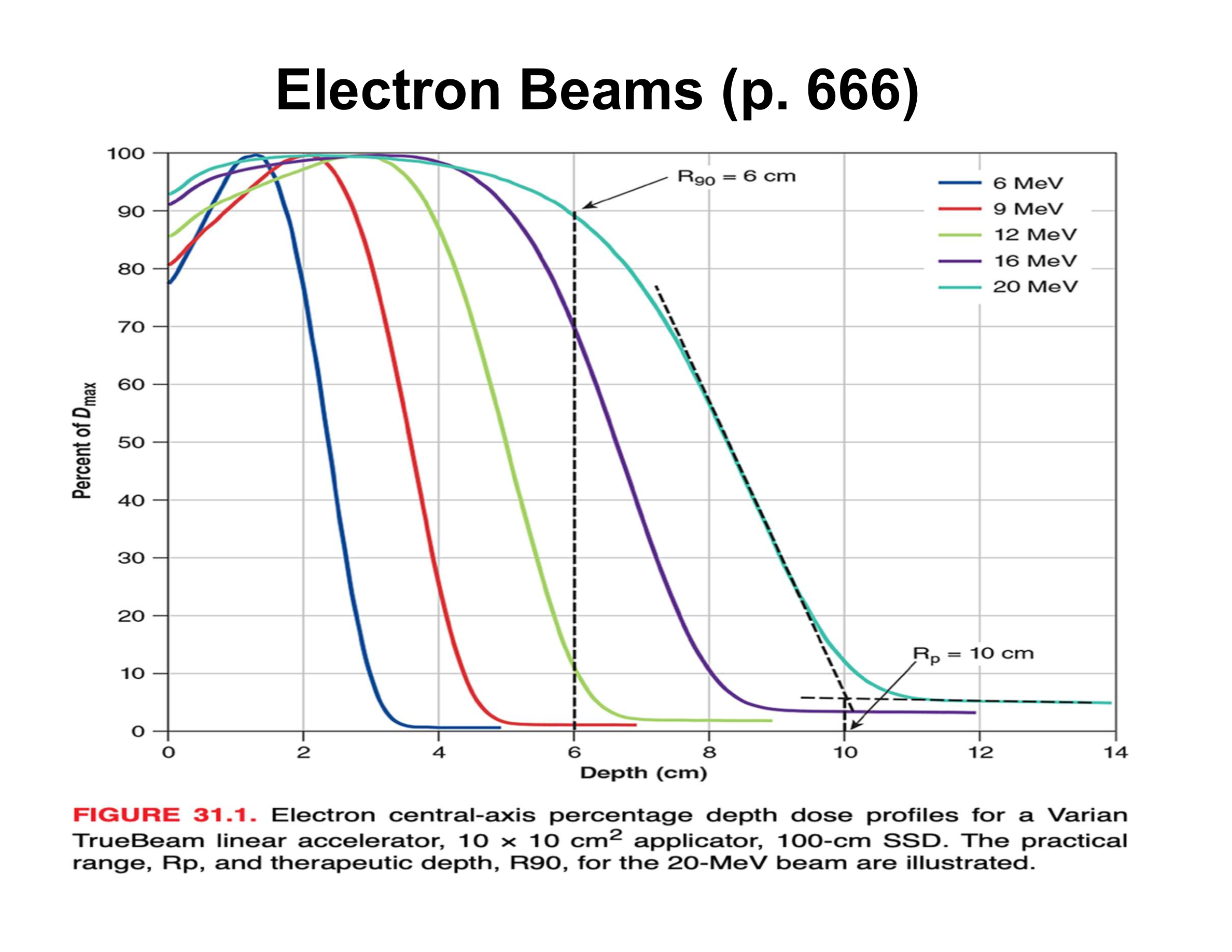
What is the rule of thumb relating energy to R90?
Energy (MeV) ≈ 3.3 × R90 (cm)
What is the optimal target–critical structure separation for electrons?
Separation (cm) ≈ Energy (MeV) / 5
Why is electron depth dose machine-dependent?
Differences in scattering foils, chambers, applicators, and geometry require machine-specific measurements
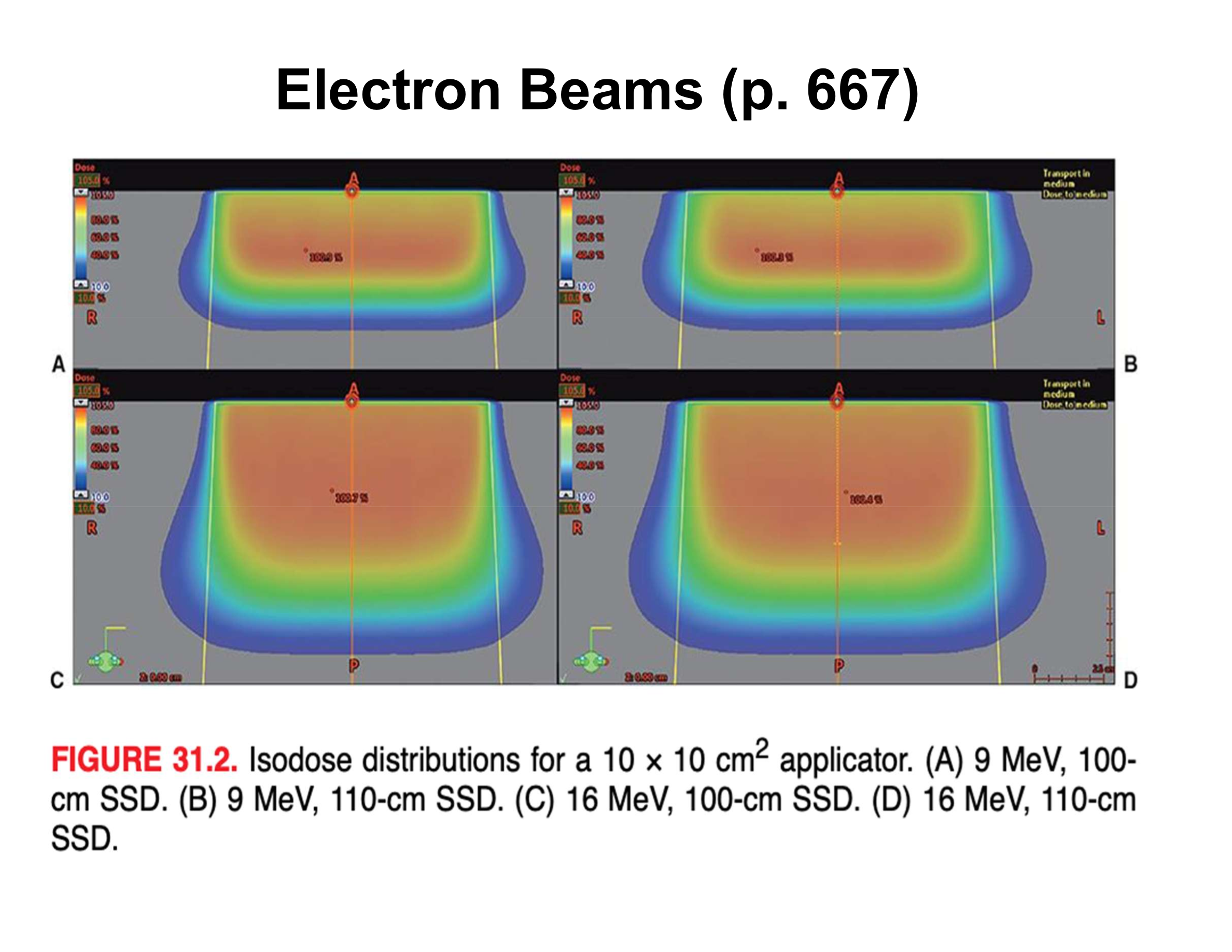
How does electron surface dose compare to MV photons?
Higher surface dose, making electrons ideal for superficial targets
How do lower-energy electrons affect surface dose and buildup?
More scattering, steeper buildup, and lower surface dose
Why is bolus used with low-energy electrons?
To increase surface dose, but it reduces therapeutic depth
What is bremsstrahlung contamination in electron beams?
Residual X-ray dose after electrons stop; typically <5% of max dose and clinically insignificant
How does electron energy affect surface penumbra?
Lower-energy electrons scatter more, producing a broader angular spread and larger surface penumbra
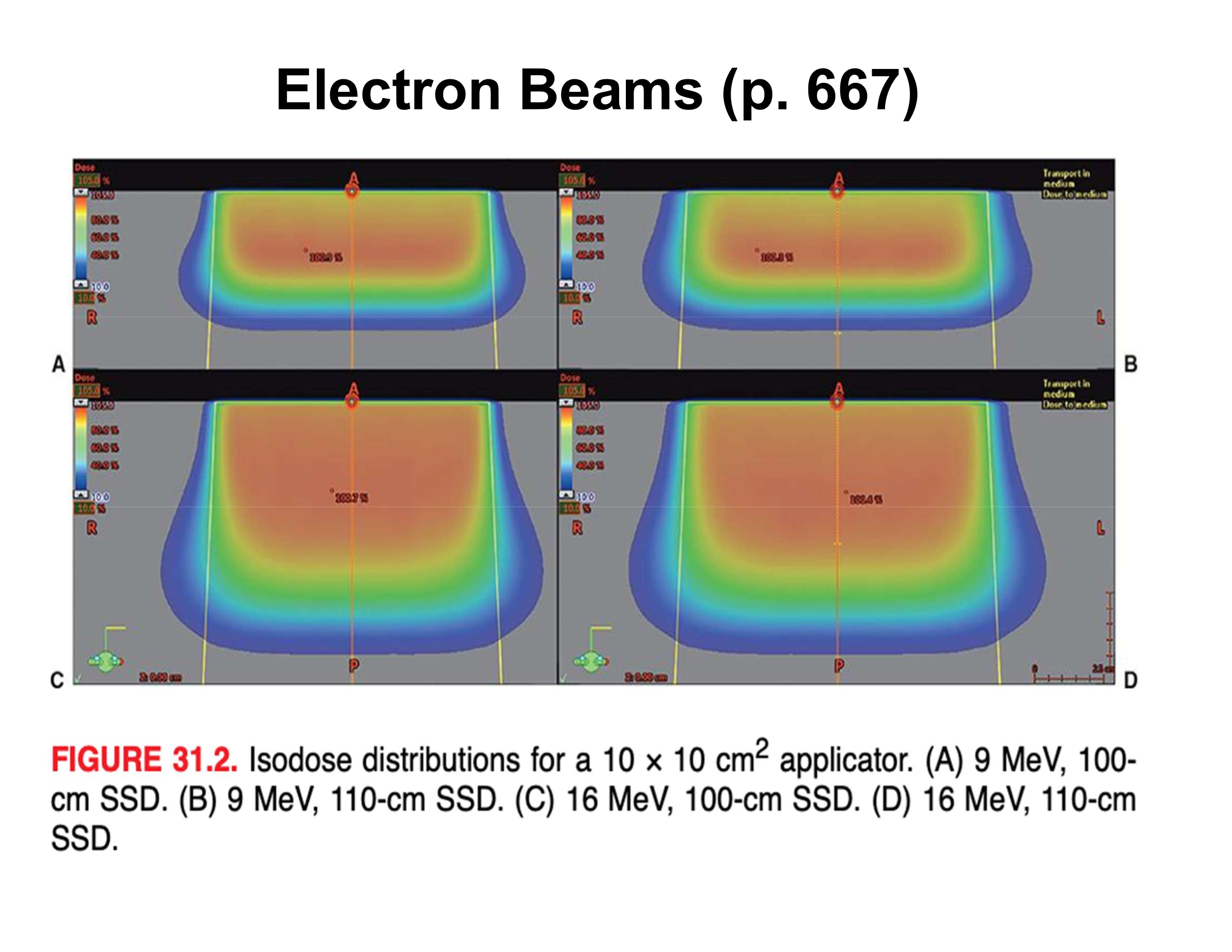
Why does a 9 MeV beam have a wider surface penumbra than a 16 MeV beam?
Increased scattering in the treatment head and air above the cutout
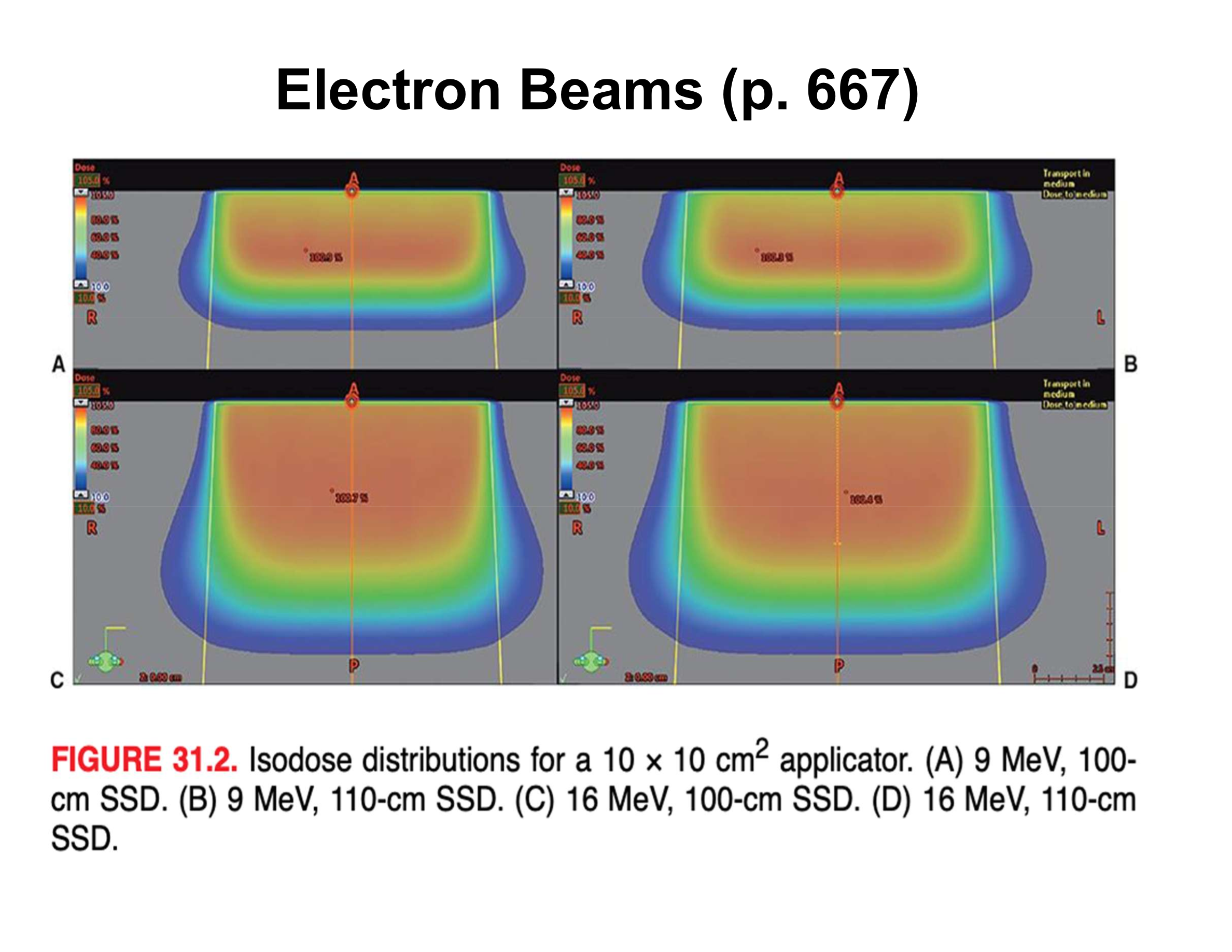
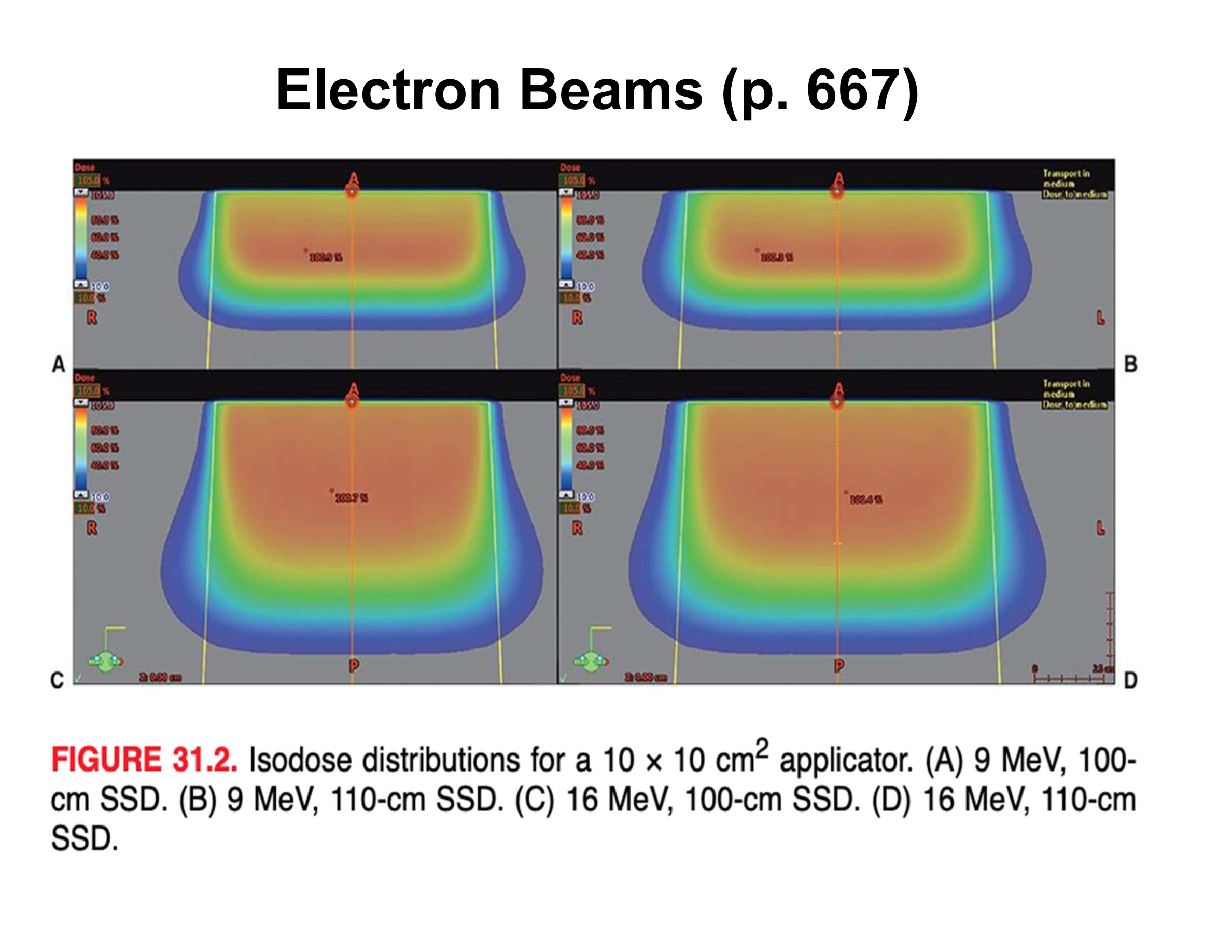
What is the effect of increasing SSD from 100 cm to 110 cm on surface penumbra?
Air gap increases ~3×, resulting in ~3× wider surface penumbra
Flashcard 5
Why does increased SSD have less effect at R100 and R90 depths?
Phantom (patient) scattering dominates over air-gap scattering at depth
What is the key clinical rule when creating electron cutouts?
Always include a dosimetric margin beyond the visible target
If target size at skin is 8 cm, what beam portal size is ≥ 10 cm, adjusted based on nearby critical structures
How are electron fields shaped clinically?
Using an electron applicator (cone) plus a patient-specific insert (cutout)
How are critical normal tissues shielded during electron therapy?
Using high-Z materials (lead, tungsten), sometimes placed directly on the patient
Why is surface shielding sometimes needed at extended SSDs?
The applicator cannot be placed close enough, so lead sheets are used on the patient
What are key characteristics of electron backscatter?
Increases with higher-Z materials, is greater at lower energies, and has low mean energy
What does increasing SSD affect for electron beams?
Penumbra, depth dose shape (small effect), and dose output (MU)
Why do small electron fields behave poorly at extended SSD?
Loss of lateral scatter equilibrium and increased scattered low-energy electrons from aperture edges
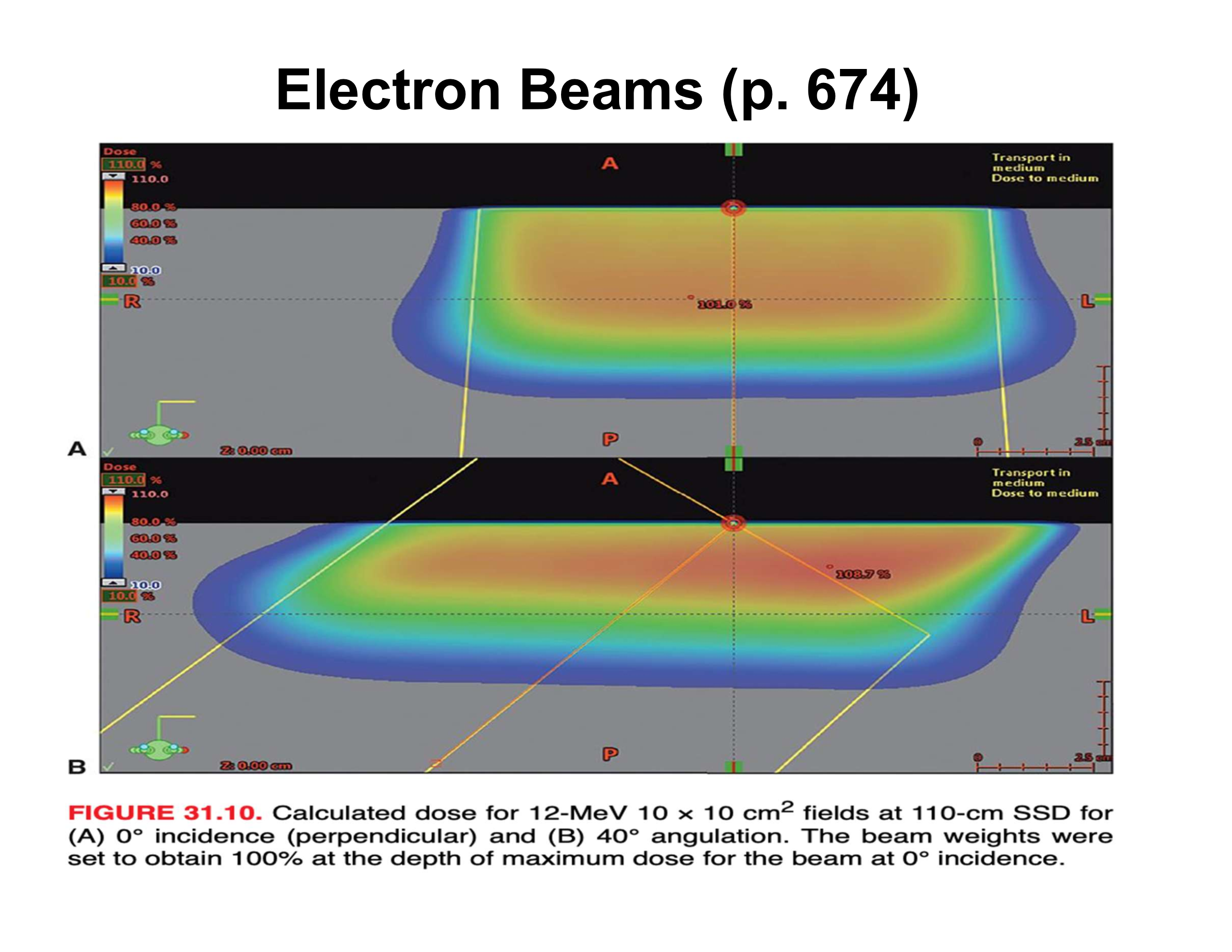
What are the three main effects of oblique electron beam incidence?
Reduced penetration, increased Dmax, asymmetric penumbra
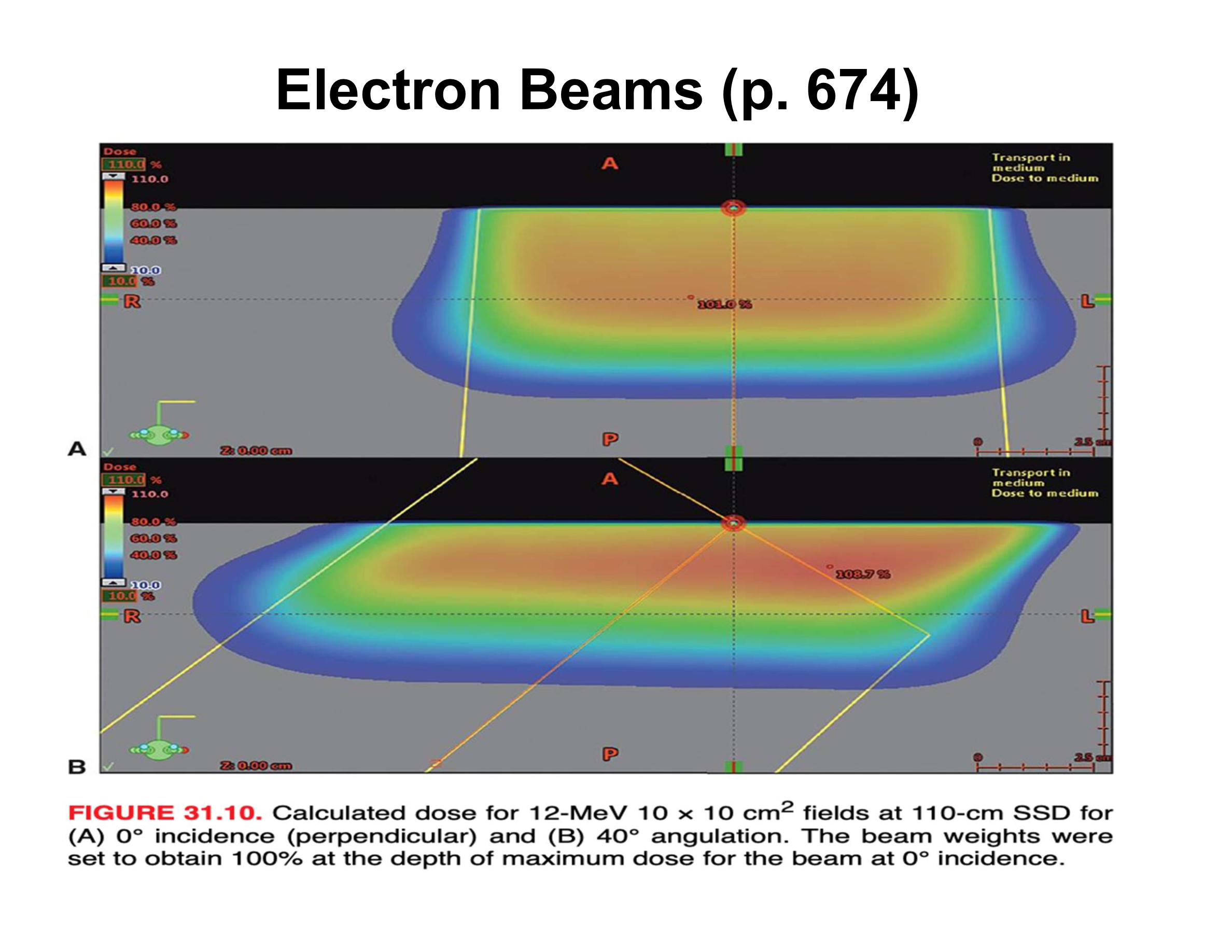
How does oblique incidence affect therapeutic depth relative to the skin surface?
Therapeutic depth decreases as beam angle increases
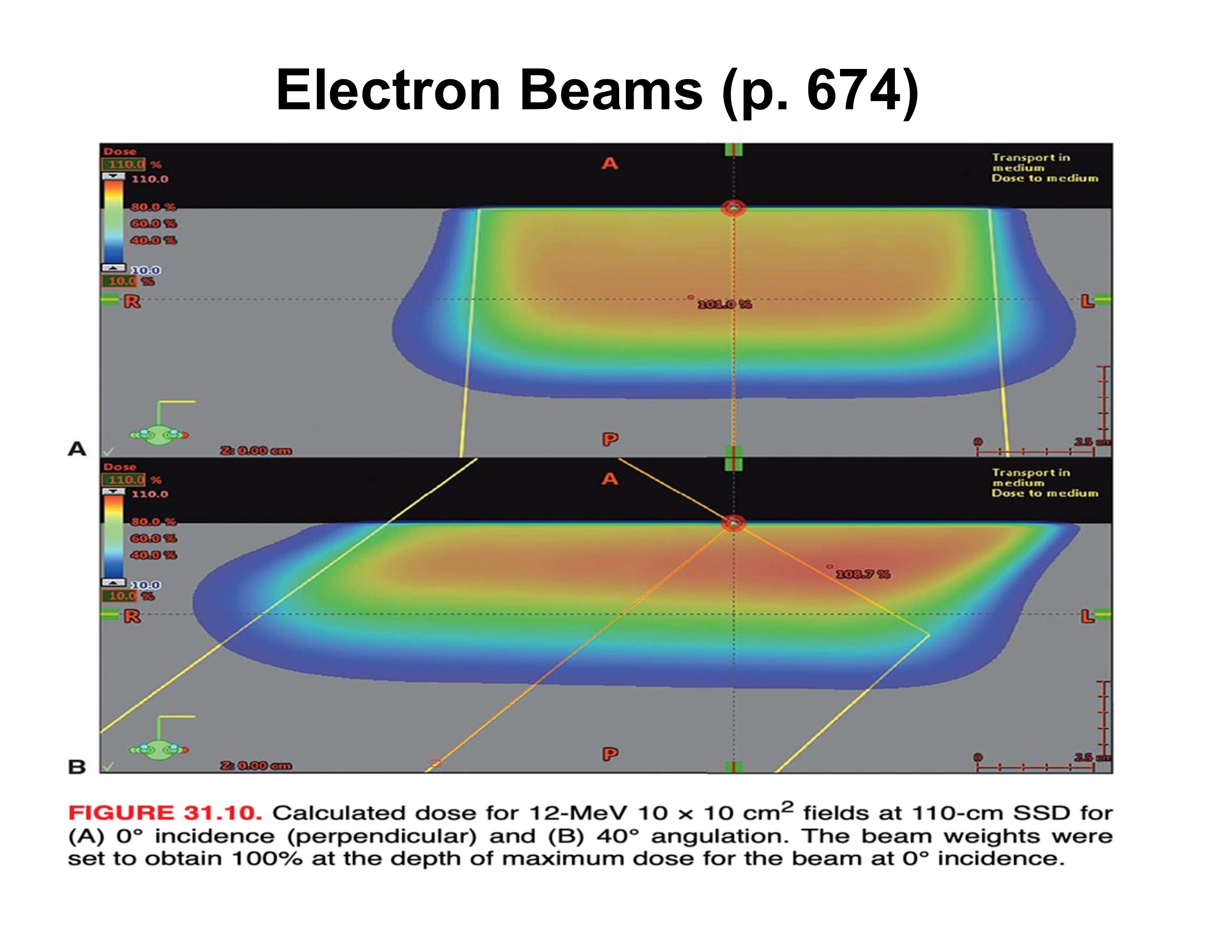
Why does Dmax increase with oblique incidence?
Electrons scattered from upstream phantom material are directed toward the central axis
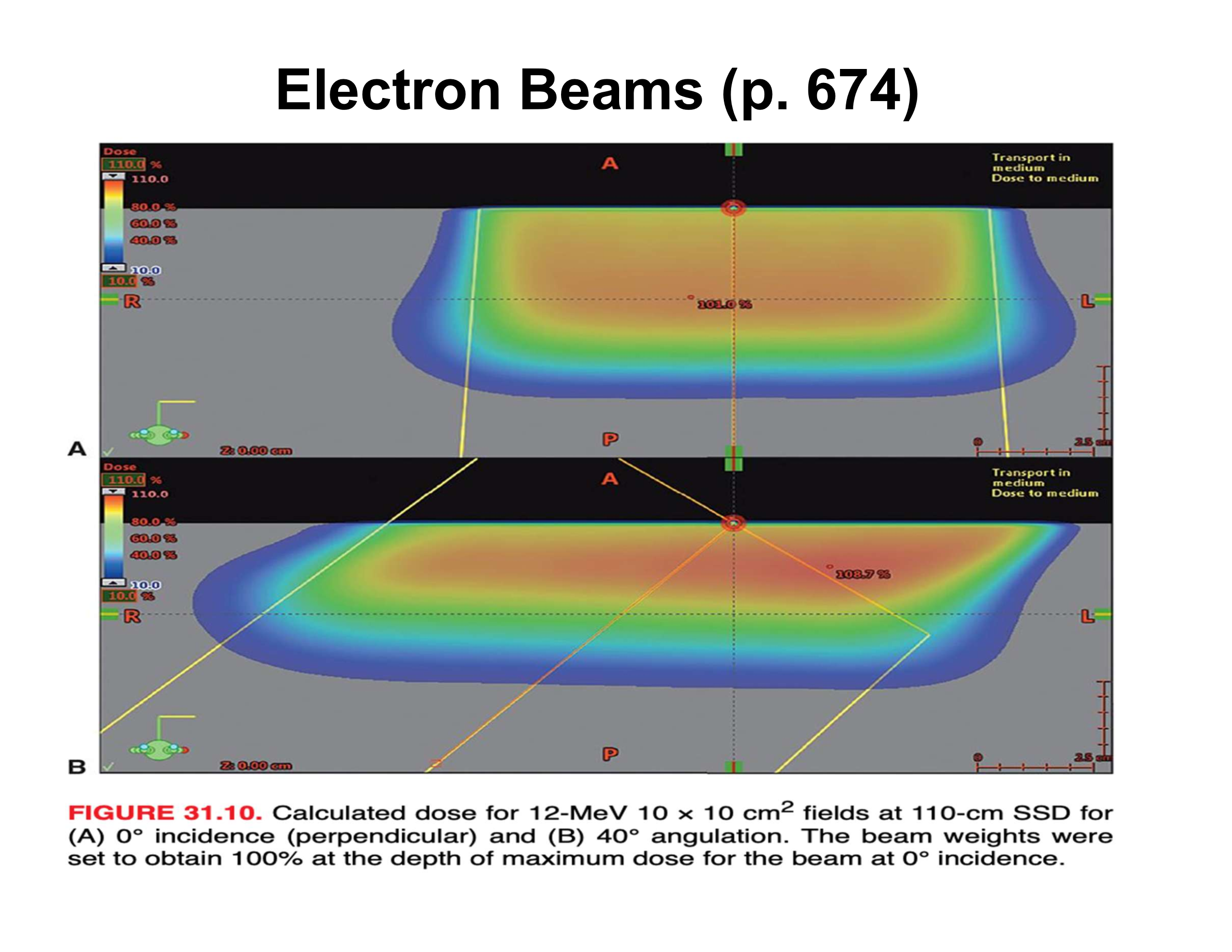
How does penumbra change with oblique incidence?
Upstream side is sharper; downstream side is broader
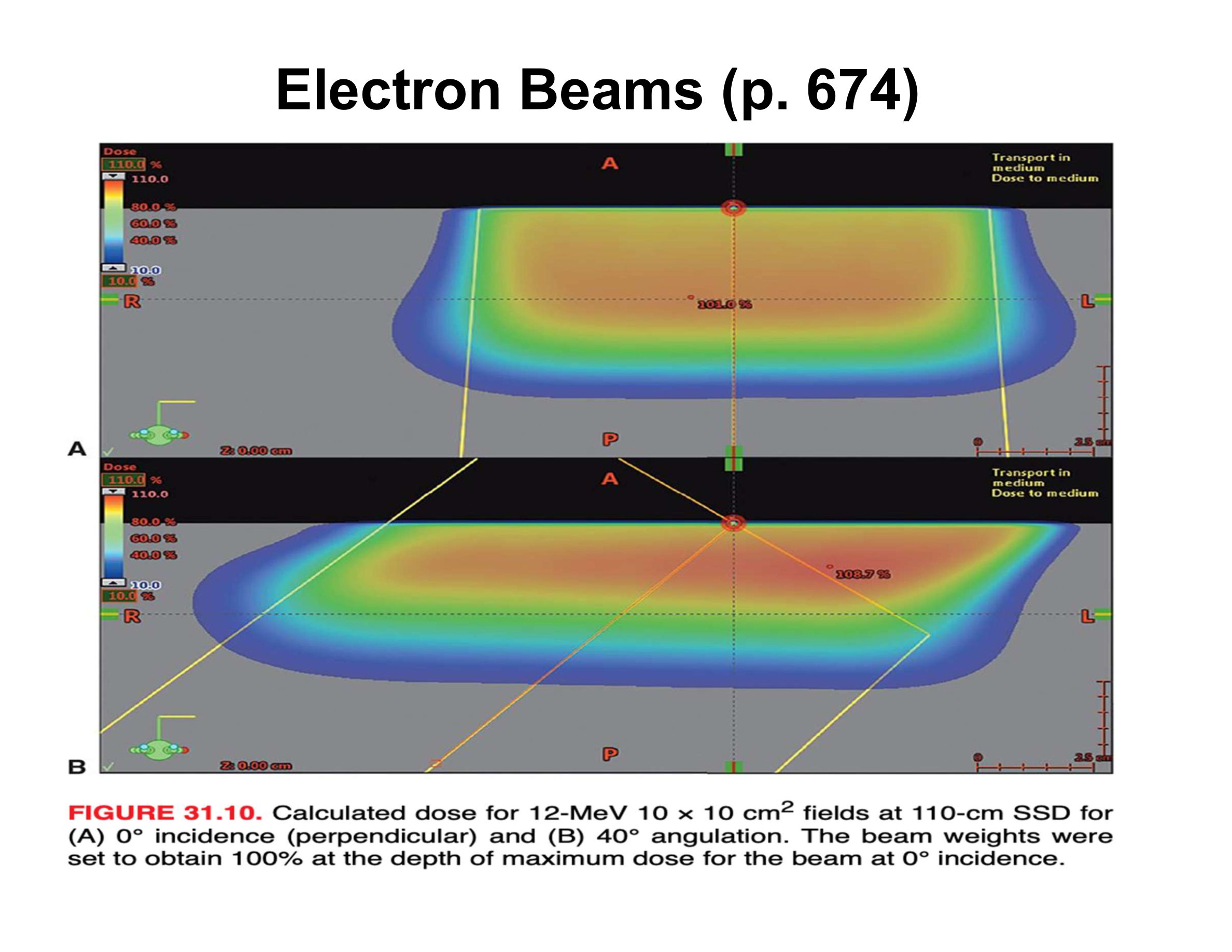
How does central-axis depth dose (along beam direction) change with increasing angle?
Decreased therapeutic depth, increased Dmax, apparent increase in practical range
Why does the practical range appear to increase along the beam axis at oblique angles?
Upstream scatter deposits dose early, downstream scatter deposits dose later, creating an illusion of longer range
What happens to surface-based depth dose as beam angle increases?
Higher dose at shallow depths and reduced penetration relative to skin
Why are patient surfaces problematic for electron therapy?
They are rarely flat, causing variable penetration and dose distortion
How do rounded patient surfaces affect electron penetration?
Greater penetration where incidence is near normal; reduced where surface slopes away