haemoglobin- mass transport in animals
1/58
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
59 Terms
describe the structure of proteins (5)
polymers of sequences of amino acids which join in a condensation reaction to form peptide bonds. primary structure is the sequence of amino acids. secondary is the folding of the polypeptide, making a beta pleated sheet or alpha helix due to hydrogen bonds. tertiary structure is the folding due to hydrogen and disulphide bonds. quaternary structure is the folding due to hydrogen and disulphide bonds
what elements are proteins made up of
carbon, hydrogen, oxygen, nitrogen, sulphur
what does haemoglobin ( a transport protein ) carry?
oxygen
what is the monomer making up proteins
amino acids
how many naturally occurring amino acids are there
20
structure of amino acids
a carboxyl group -cooh. an amine group- nh2. a variable group- r
structure of haemoglobin
globular protein which is made up of 4 peptide chains, each containing one haem group. its structure is curled up so that hydrophilic side chains face outwards and hydrophobic side chains face inwards. this makes haemoglobin soluble and is therefore good transport for blood
where is haemoglobin transported
red blood cells
why is haemoglobin soluble
structure is curled so that hydrophilic side chains face outwards and hydrophobic side chains face inwards. therefore it is good for transport in the blood
the structure of haem is the same in all haemoglobin, but the globin chains vary between species. what causes this change in protein structure between species?
different primary sequences of amino acids lead to different hydrogen bonding in secondary structure and different ionic and disulphide bonding in tertiary and quaternary structures
reversible reaction for haem group combining with oxygen molecule
Hb + 4O2 = 4Hb(O2)
what is partial pressure (po2)
a measure of oxygen concentration- pO2 is high in the lungs and lower in body tissues like muslce
what is affinity
haemoglobins affinity for oxygen depebds on the pO2. high affinity means oxygen binds easily
oxygen associates (loads) with haemoglobin to form oxyhaemoglobin where there is a _____ pO2?
high
oxygen dissociates (unloads) from oxyhaemoglobin where there is a _____ pO2?
lower, e.g muscles
what is the saturation
how much oxygen being carries
haemoglobin binds _____ with oxygen so is a good transporter of oxygen
reversibly
what is the affinity of haemoglobin for oxygen at a gas exchange surface? e.g. lungs, gills
high pO2 in the medium (water of ==r air). haemoglobin has a high affinity for oxygen so oxygen associates/loads with Hb
what is the affinity of haemoglobin for oxygen at respiring tissues? e.g. muscles
low pO2 in cells. haemoglobin has low affinity for oxygen. oxygen unloads from haemoglobin. high pCO2 causes haemoglobin to change shape and unload oxygen
what is an oxygen dissociation curve
relationship between the saturation of haemoglobin with oxygen and the partial pressure of oxygen
what is the % saturation of haemoglobin
the percentage of haemoglobin associated with oxygen at a given pO2
what does % saturation of Hb depend on?
pO2 of the environment
explain how oxygen is loaded, transported and unloaded in the blood
haemoglobin carries O2- has a high affinity of O2. haemoglobin found in red blood cells. loading takes place in the lungs at high oxygen concentration. oxygen unloads at respiring cells/tissues at low oxygen concentration. unloading linked to higher carbon dioxide concentration
Hb reaches nearly 100% oxygen saturation even when partial pressure is lower than atmospheric partial pressure of 21kPa. why is this and what is the advantage of this?
in alveoli, pO2 is less than 21kPa as alveolar air contains a lot of water vapour and high CO2. pO2 is around 15kPa which is still high enough to saturate almost 100% of the Hb
the blood system carries red blood cells through a PV into the heart, then pumped out to body tissues. explain why Hb does not unload its oxygen before it reaches the capillaries in the tissues
walls of arteries, veins and arterioles are too thick to allow gaseous exchange. the pO2 around the red blood cells remains constant so the Hb remains saturated
if the curve on an O2 dissociation graph is further to the left, what is the affinity?
higher affinity- holds more oxygen
if the curve on an O2 dissociation graph is further to the right, what is the affinity?
lower affinity- spends more oxygen
why is it important for a baby to develop haemoglobin after birth
as they begin to breathe air and become more active, it needs to release oxygen more readily at tissues. doesn’t need to take up O2 at low pO2 as the air has a pO2 of approx 21kPa
what is the bohr effect
a curve to the right of a normal dissociation curve
why does the bohr effect occur
high concentration of carbon dioxide and an increase in temperature
in active tissues, what percentage of oxygen is unloaded
45%
in active tissues, more than 45% of oxygen is unloaded. an active tissue is respiring and producing CO2. why is more oxygen unloaded?
haemoglobin has a reduced affinity, therefore higher dissociation for oxygen in the presence of carbon dioxide
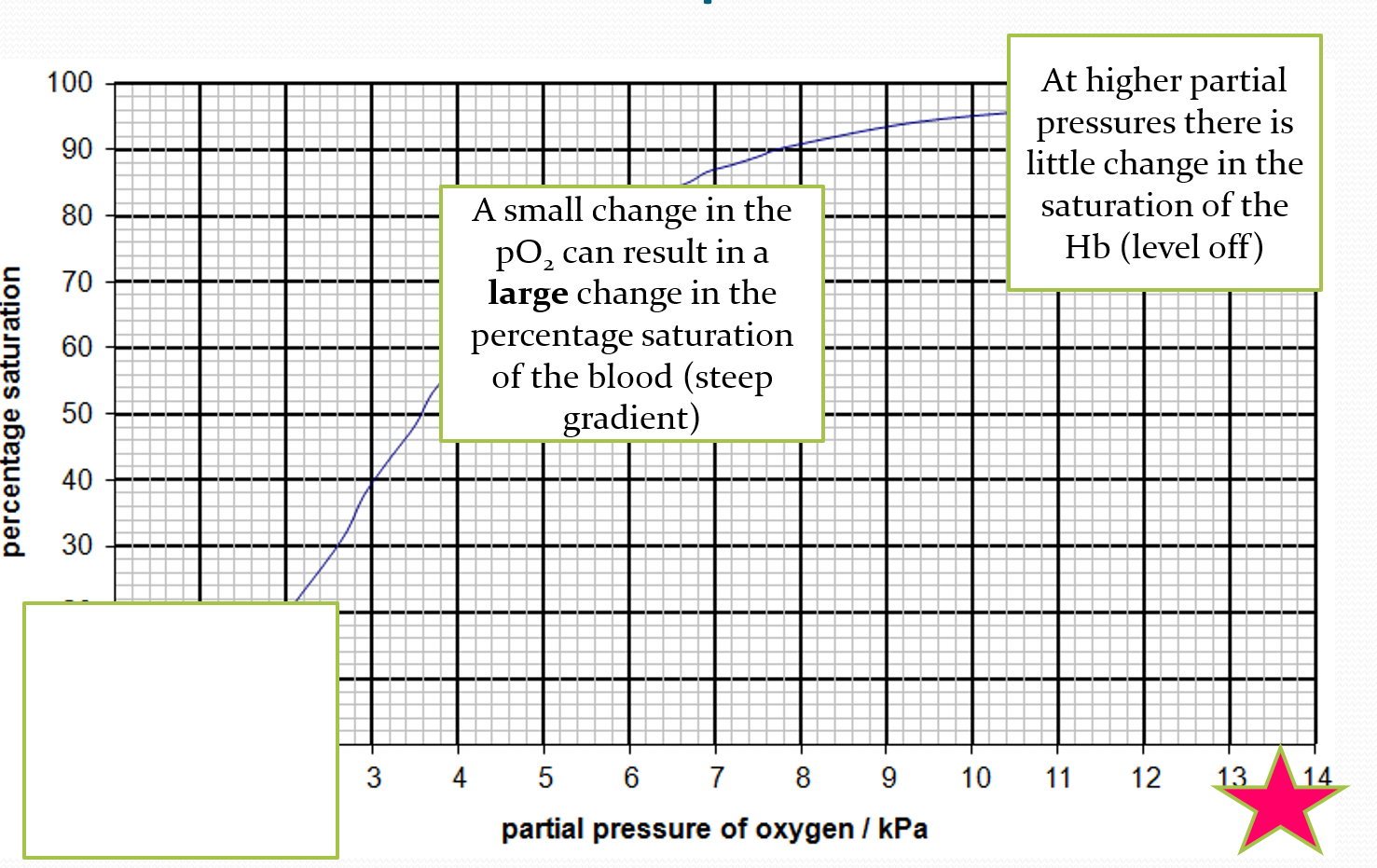
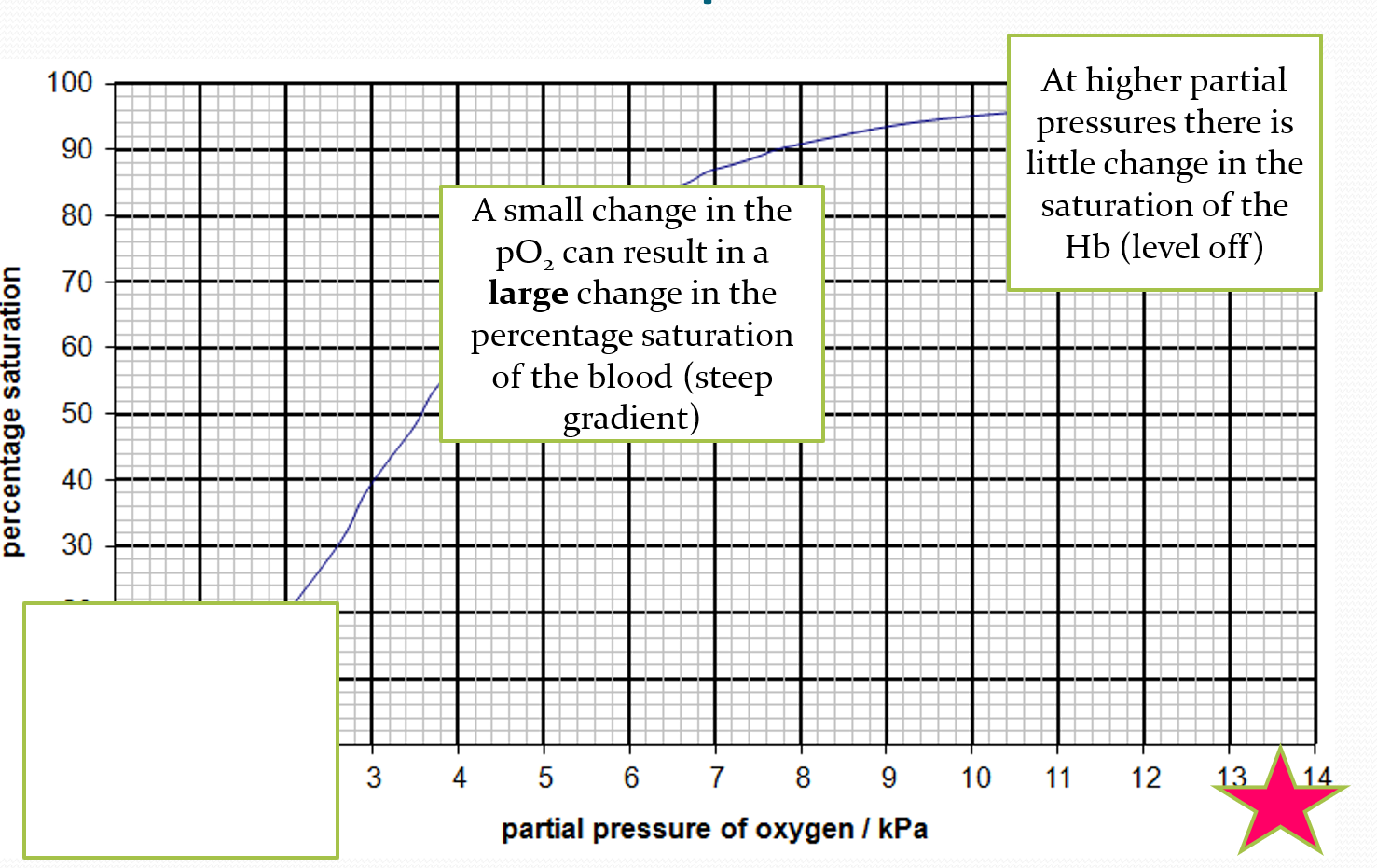
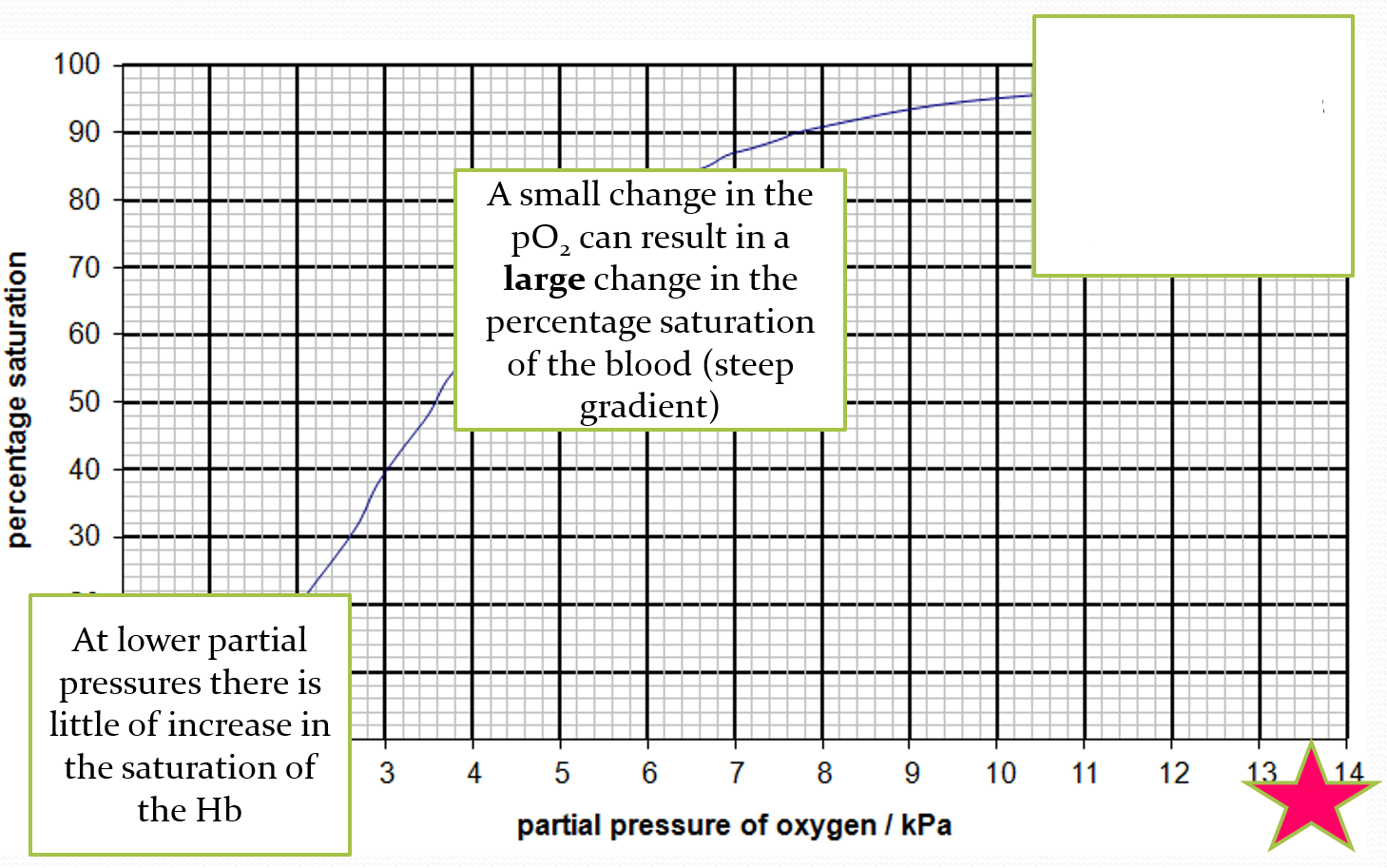
DESCRIBE what is happening at the bottom of the oxygen dissociation curve
at lower partial pressure, theres a little increase in saturation of haemoglobin
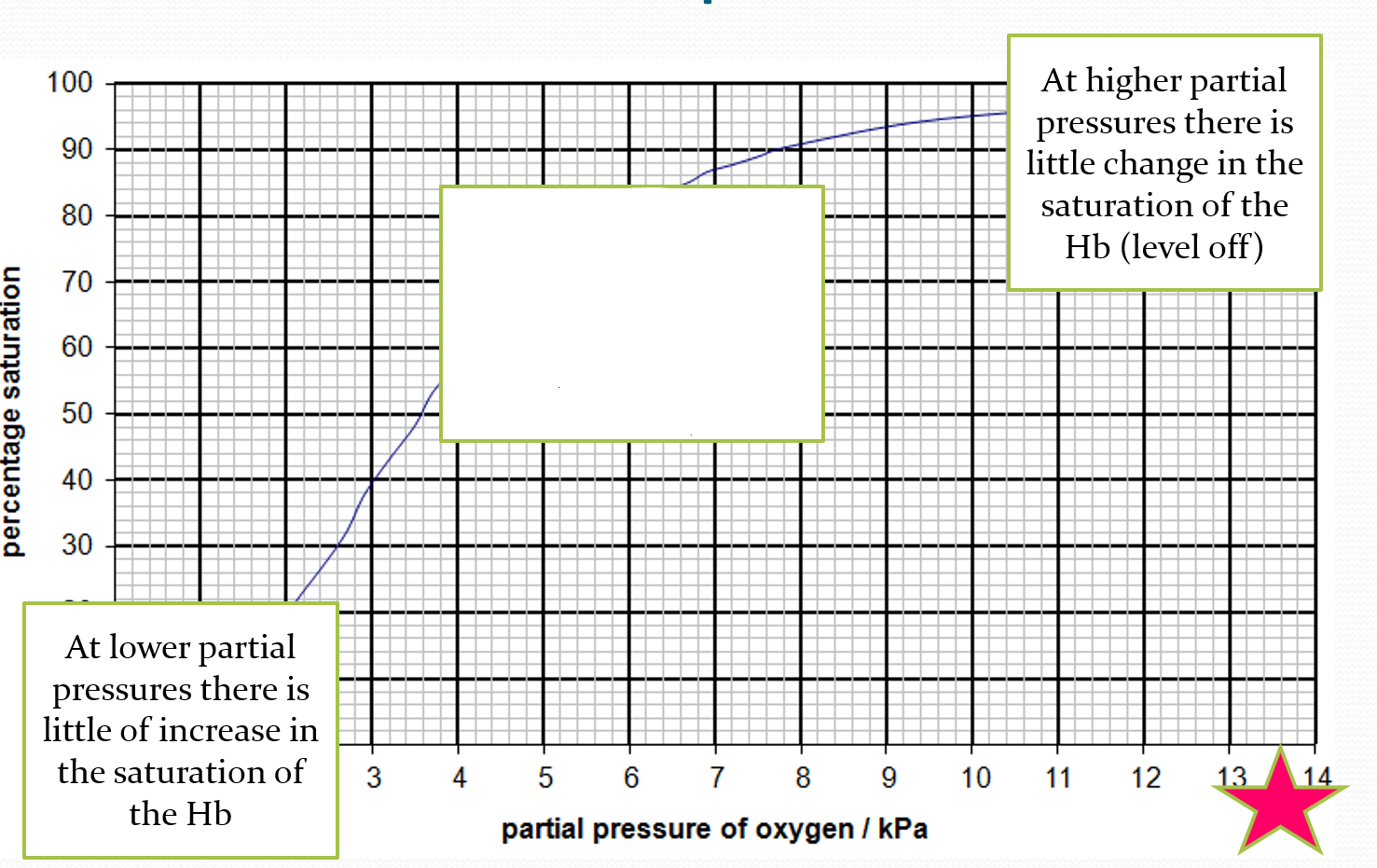
DESCRIBE what is happening in the middle of the oxygen dissociation curve
small change in pO2 can result in a large change in the percentage saturation of the blood- steep gradient
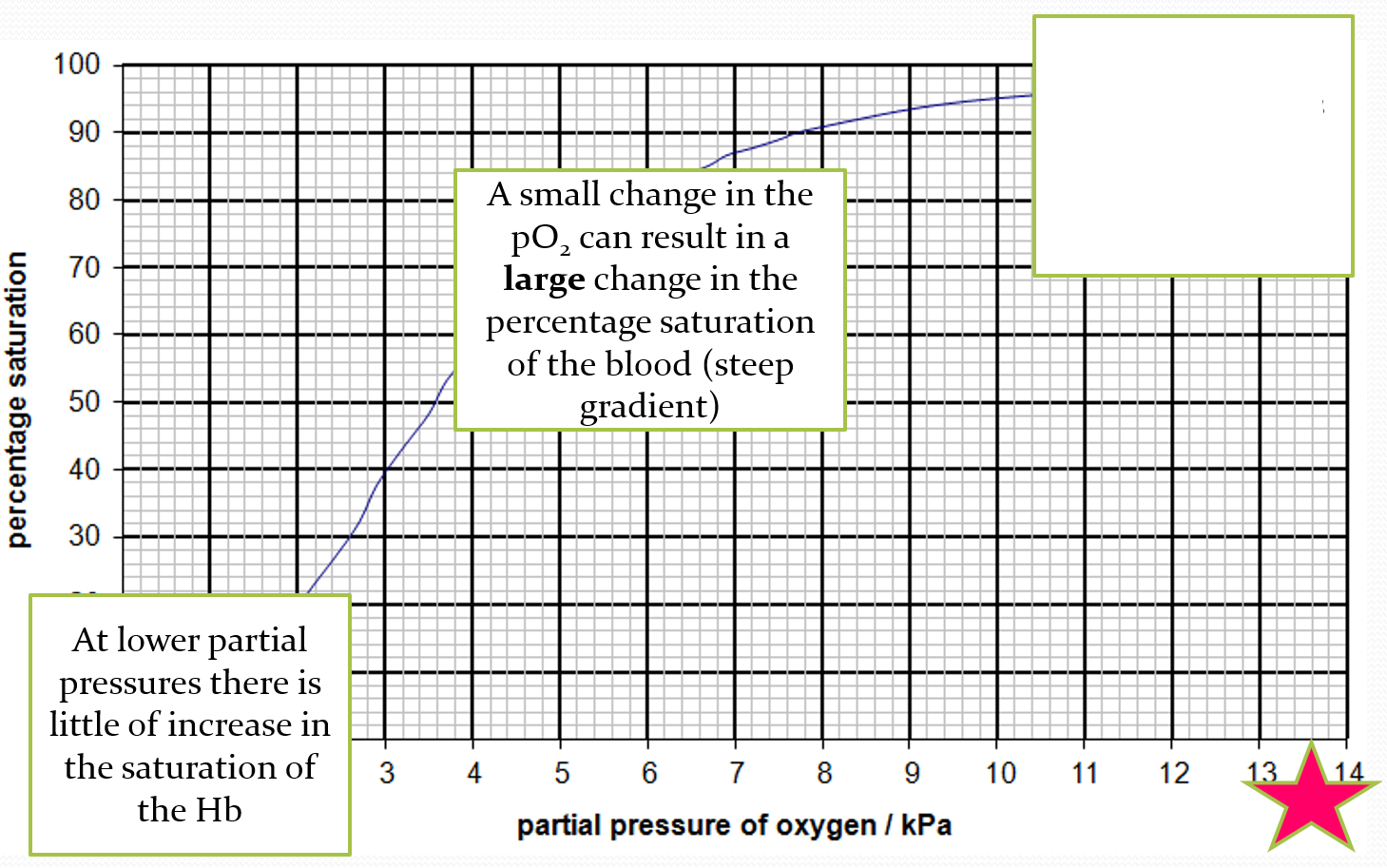
DESCRIBE what is happening at the top of the oxygen dissociation curve
at higher partial pressures, there is little change in the saturation
EXPLAIN what is happening at the bottom of the oxygen dissociation curve
first molecule of oxygen combines with haemoglobin and slightly distorts it. the joining is quite slow
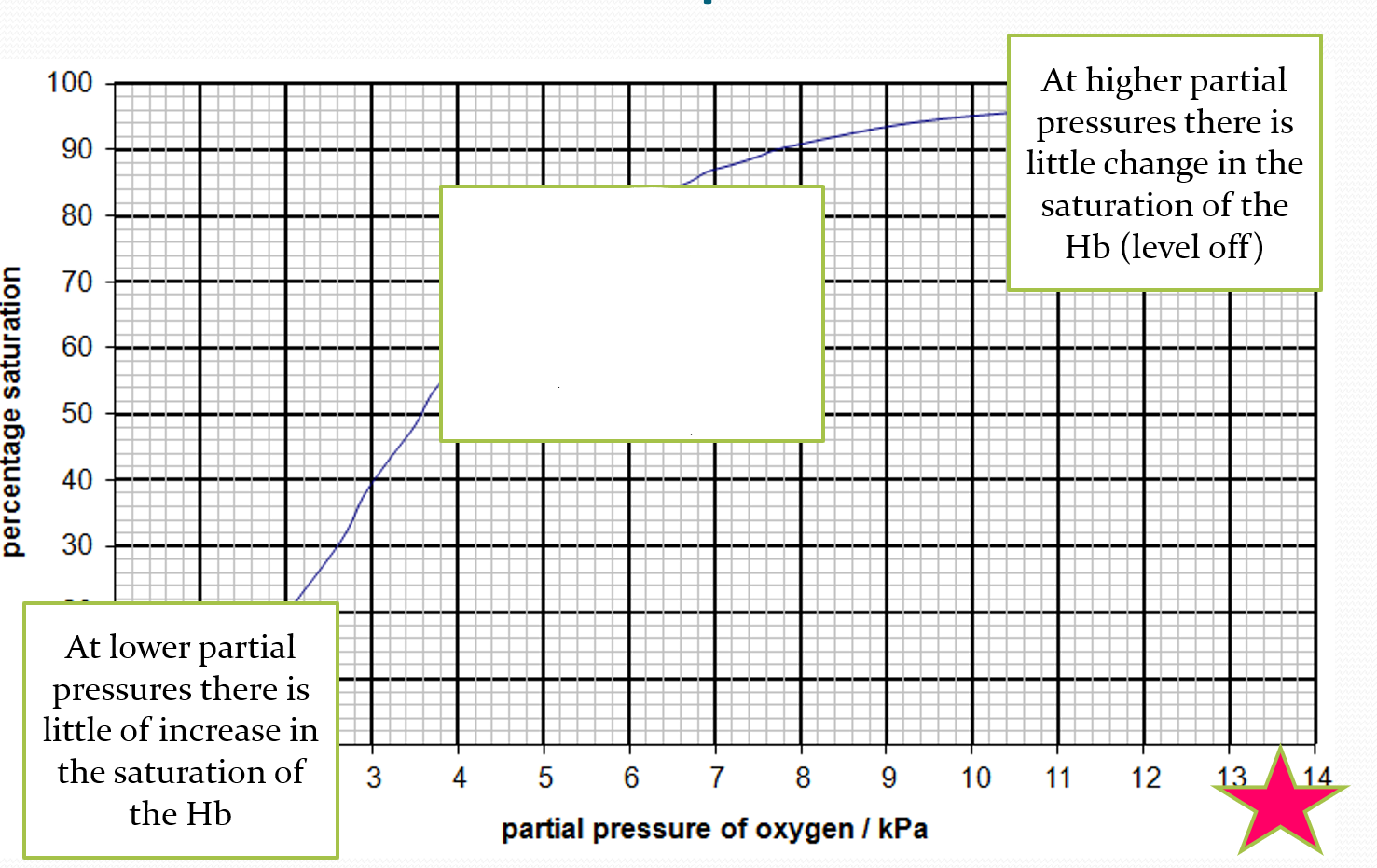
EXPLAIN what is happening at the middle of the oxygen dissociation curve
after the haemoglobin has changed shape a little, it becomes increasingly easy for the second and third oxygen to join. this is called cooperative binding
EXPLAIN what happens at the top of the oxygen dissociation curve
flattens off at the top because joining the fourth oxygen is more difficult- fewer sites available to bind to
what is the bohr effect
at lower PH values, oxygen dissociates from Hb more readily. this is useful in the tissues as the cells are respiring aerobically faster so they need more oxygen. this increases the rate of oxygen disassociation and the curve shifts to the right. this means the saturation of blood with oxygen at a given partial pressure is lower because more oxygen is being released
effects of carbon dioxide on the lungs
conc of CO2 is low because it diffuses readily into alveoli. affinity for oxygen is increased. this means that oxygen unloaded more readily with haemoglobin
effects of carbon dioxide on the tissues
concentration of CO2 is high because respiration releases CO2. the affinity of haemoglobin for oxygen is decreased to oxygen loads more readily to provide oxygen for aerobic respiration
effects of carbon dioxide on protein structure of Hb
co2 is acidic. lowers pH which alters the structure of the protein, especially the bonding. change in pH alters the affinity of Hb for oxygen by slightly changing the shape of the molecule. lower pH means higher level of co2 in the tissues. change in shape lowers Hb affinity for oxygen, so O2 more readily unloads/dissociates
the effect of carbon dioxide concentration on the function of haemoglobin
haemoglobin gives up its oxygen more readily at high partial pressure of co2. this enables more oxygen to get to cells that are respiring at a high rate. when cells respire they produce carbon dioxide, therefore increasing the pco2
exam q: describe and explain the effect of increasing carbon dioxide concentration on the dissociation of oxyhaemoglobin
more oxygen unloading by decreasing blood pH
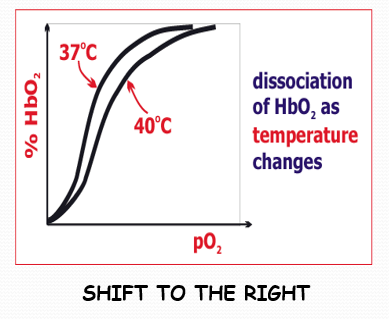
how does blood temperature affect dissociation
increased blood temperature reduces haemoglobins affinity for O2. therefore, more oxygen is delivered to warmed-up tissue. more oxygen is loaded from haemoglobin at tissue level
an increase in blood temperature makes the dissociation curve shift in which direction?
to the right
how does the size of an animal affect dissociation
smaller animals have a high metabolic rate as they need oxygen to be released readily in the tissues. therefore their haemoglobin has a lower affinity for oxygen
how does altitude affect dissociation (e.g. llamas)
live at higher altitudes where there is lower partial pressure of oxygen. haemoglobin has a higher affinity for oxygen than similar lowland animals so that it can bind to any oxygen that is available
how does higher altitude shift of the dissociation curve
shifts to the left
how do sand burrows (intertidal) affect dissociation
there is low partial pressure of oxygen in a burrow. need higher affinity haemoglobin as picks up oxygen more readily but releases it less readily.
which way does the dissociation curve shift for sand burrows
to the left
do organisms with less oxygen in their environment need haemoglobin with a low or high affinity?
high affinity
what does low affinity mean
dont take up oxygen easily but release it more readily
what does high affinity mean
take up oxygen very easily but dont release it as readily
why do animals with a high metabolic rate need haemoglobin with a low affinity for oxygen
to that oxygen is released more easily at the respiring tissues
what is foetal haemoglobin
oxygen dissociates from maternal haemoglobin in placenta and foetal haemoglobin loads with oxygen. curve further left
what do higher affinity haemoglobins have and what does it do
myoglobin- red pigment in slow twitch muscles adapted for aerobic respiration. it has an even higher affinity for oxygen than haemoglobin, only releasing it at very low partial pressures. it stores oxygen
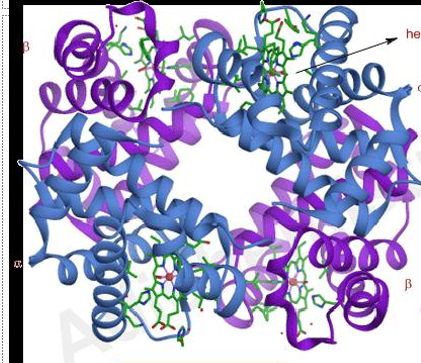
what is this
haemoglobin
what is this
myoglobin