Horticulture final exam
1/107
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
108 Terms
Geophyte
plants that survive as specialized underground storage organs
What do genotypes include
Bulbs, corms, tubers, tuberous roots, rhizomes, and pseudobulbs
Primary function of geophytes
Storage of food, nutrients, and water. Survical during adverse enviromental conditions.
Characteristics of geophyte
•Herbaceous perennials
•Shoots die down at the end of the growing season
•Dormant, fleshy organs bear buds for new shoots
Adaptions of geophyte
•Withstand adverse growing conditions
•Never physiologically dormant
•Act as “bioprocessors” sensing and responding to the environment
1.Warm–cold cycle (temperate zones)
2.Wet–dry cycle (tropical and subtropical regions)
•Clonal regeneration
Use of both sexual and asexual systems
Bulbs
specialized underground organ consisting of a short, usually vertical stem axis (basal plate) bearing at its apex a growing point or a flower primordium enclosed by thick, scale-like leaves
What type of plants are bulbs mostly produced
monocotyledonous plants (the usual plant structure is modified for storage and reprodcutions)
Classification of Bulbous Species
•Hardiness
•Hardy, semi-hardy, or tender
•Time of flowering
•Structure
•Tunicate (laminate)
•Scaly (non-tunicate)
Structure of a Typical Bulb
Hyacinth bulb with a dry and papery, tunicate (laminated) covering. Longitudinal section of a hyacinth bulb showing basal plate, bulb scales, and flower axis (like an onion).
Bulblet
a miniature bulb that forms in the axil of a bulb scale and provides a method of vegetative propagation.
Tunicate (Laminate) Bulbs
•Dry, membranous outer bulb scales are called the “tunic”
Provides protection from drying and mechanical injury
•Fleshy inner scales
Food storage
•The fleshy scales are in continuous, concentric layers, or lamina, so that the structure is more or less solid.
•Produced in onion and garlic (Allium), daffodil (Narcissus), tulip (Tulipa) and many genera in the family Amaryllidaceae.
Nontunicate (Scaly) Bulbs
•No tunic
•Scales are separate and attached to the basal plate
•Easily damaged and must be handled more carefully than the tunicate bulbs
•Must be kept continuously moist because they are injured by drying
•Contractile roots
•Represented by lily (Lilium) and Fritillaria
(Looks like and artichoke)
Can geophytes have fibrous and contractile roots.
Yes
Fibrous
formed as adventitious roots; they absorb water and nutrients and normally function for only one growing season.
Droppers
• modified stem certain geophytes use
•A new bulb that forms at the end of a stolon
•Helps to move the bulb down to its proper depth where a new bulb is formed
•Produced by tulips, trout lilies, seedling bulbs
Bulb Life Cycle
reproductive stage
Vegatative stage
Bulb storagte conditions
•Store in a cool, dry place to prevent premature sprouting
•First allow them to dry in an area with good air circulation to remove excess moisture
•Temperature range for storing is between 40°F (4°C) and 50°F (10°C)
•Avoid areas with extreme temperature fluctuations or high humidity
•Containers that allow for air circulation, such as mesh bags, paper bags, or boxes with ventilation holes
•Regularly check for signs of rot or dehydration
Propagation of Bulbs
1.Normal offsets
2.Underground stem bulbets
3.Aerial stem bulbets
Bulbil
Bulbet Formation on Stems
Tunicate Bulbs
Propagate by Twin Scaling
How to Propagate via Twin-Scaling
1.Cut bulb into sections each of which contain two scale sections and a portion of the basal plate
2.Optional: fungicide
3. Place bulb pieces in plastic bags with moistened perlite or vermiculite (14 perlite: 1 water)
4.Close bag tightly and place in a dark place at room temperature for 7 week
How to Propagate via Scaling
•Same as twin-scaling, except:
•Break off scales individually
•Scales do not need to contain part of the basal plate
•After bulbets develop, they may need at least 8 weeks of chilling at 35-40 degrees F
Propagation of Bulbs by Scoring & Scooping
1.Score or scoop basal plate
2.Callused cut side down in dry sand
3.Incubate in dark, high humidity
4.Planted in ground in fall
5.Leaves produced following spring
6.Flower after 4-5 years
Bulb Cuttings
1.A mature bulb is cut into a series of 8 to 10 vertical sections, each containing a part of the basal plate.
2.These sections are further divided by sliding a knife between each third or fourth pair of concentric scale rings and cutting through the basal plate.
3.Each of these fractions makes a bulb cutting, and consists of a piece of basal plate and segments of 3 or 4 scales.
Leaf Cuttings
1.Leaves are taken when they are well developed and green.
2.An entire leaf cut from the top of the bulb may in turn be cut into two or three pieces.
3.Each section is placed in a rooting medium with the basal end several inches below the surface.
4.Within 3 to 4 weeks, small bulblets form on the base of the leaf, roots develop, and the bulblets are planted in soil
Corm
the swollen base of a stem axis enclosed by a dry, scale-like tunic. It has
•Contractile roots
•A solid stem structure with distinct nodes and internodes
•Tunic to protect against injury and water loss
Bulb vs. Corm
Bulb Corm
-Leaf, stem, AND flower tissue -Stem tissue only
-Nodes & internodes NOT distinct -Distinct nodes & internodes
-Scales -Solid, no scales
Corm Propagation
1.Sexual (seeds)
2. New corms
3.Cormels
◦Miniature corms that develop between the old and the new corms
4.Corm division
◦Large corms
◦Each section must contain a bud
5.Tissue culture
Tuber
•special kind of swollen, modified stem with nodes and internodes
•Functions as a storage structure as well as an organ of vegetative propagation
•“Tuberous stem”
•highly nutritious and composed of enlarged parenchyma-type cells
Tuber Growth Patterns
•Potato (Solanum tuberosum) serves as a good example
•The potato tuber is a storage structure that is produced in one growing season, remains dormant during the winter, and then functions to regenerate new shoots the following spring.
•After a new seasonal cycle begins, the shoots utilize the stored food in the old tuber, which then disintegrates
•As the main shoot develops, adventitious roots are initiated at its base, and lateral buds grow out horizontally into the soil to produce elongated, etiolated stems (stolons)
Tuberization
•the biological process that leads to tuber formation
•This process is associated with
•Short or intermediate daylengths
•Reduced temperatures (particularly at night)
•High light intensity
•Low mineral content
•Increased cytokinins and inhibitors (ABA)
•and reduction in gibberellin levels in the plant
Tuber Propagation: Division
•Most common propagation method for tuber-producing plants
•Tubers are cut into sections, usually two buds per piece.
“Seed” potato
•Seed potato: horticultural term applied to potato tubers when used for propagation
Tuber Propagation: Tubercles
•Aerial tubers (tubercles) produced in the axils of the leaves
•These tubercles are removed in the fall, stored over winter, and planted in the spring.
•Uncommon
•Begonia evansiana, the cinnamon vine (Dioscorea batatas), and wild yam (Dioscorea sp.)
Geophytes: Tuberous Roots
•Tuberous roots are thickened underground structures that are modified roots for food storage.
Enlarged fleshy root with shoots produced at one end and roots at the other. Biennial structure.
Tuberous Root Growth Pattern
biennial
•produced in one season
•Shoot die back in the fall à dormancy
•The following spring, buds from the crown produce new shoots, which utilize the food materials from the old root during their initial growth.
•The old root then disintegrates
•New tuberous roots are produced, which maintain the plant through the following dormant period
Crown Divison
(a) Dormant plants are lifted from the field and soil is removed by washing. (b) Plants are divided by cutting through the crown so that each division has (c) a section of the crown bearing several buds. (d) A high-grade division has four or more buds (eyes).
Adventitious Shoots
•The fleshy roots of a few species of plants have the capacity to produce adventitious shoots if subjected to the proper conditions.
“Slips”
•Adventitious roots develop from the base of these adventitious shoots. After the slips are well rooted, they are pulled from the parent plant and transplanted into the field
Tuberous Root Propagation
(a) Sweet potato (Ipomoea) is a typical fleshy root with shoots arising only from the crown at the proximal end. (b) To increase the number of propagated plants per fleshy root, growers place fleshy roots in sand under protective culture to produce (c) “slips” (shoots with adventitious roots) that can be removed and treated like rooted cuttings.
Rhizomes
•A modified stem structure in which the main axis of the plant grows horizontally at or just below the ground surface.
•It is distinguished from a stolon because it also tends to be modified as a storage organ
Rhizome Examples
•Bamboo
•Sugarcane
•Banana
•Grasses
•Iris (some)
•Lily of the valley
•Low bush blueberry
•Ferns
What are the two different types of rhizomes
Leptomorph and Pachymorph
Characteristics of a leptomorph rhizome.
•Monopodial, indeterminate growth pattern
•Continuous growth in length from the terminal apex and from lateral branch rhizomes.
•This type does not produce a clump but spreads extensively over an area.
•Examples: Japanese spurge (Pachysandra), most bamboos, and a number of grasses.
Characteristics of a pachymorph rhizome
•Sympodial, determinate growth pattern where
•The apical node terminates in a flowering stem.
•Subsequent growth continues from a lateral bud
•Plants with this growth pattern tend to form slow-growing clumps radiating from the initial plant.
•Examples: Solomon’s seal (Polygonatum), Iris, and ginger (Zingiber).
Rhizome Growth Patterns
(a) leptomorph rhizomes spread extensively over an area and do not form clumps
(b) pachymorph rhizomes have determinate growth ending in a flowering stem
(c) Bermuda grass shows an indeterminate leptomorph growth pattern with shoots produced at each node and an apex with continual growth.
(d) Iris shows a determinate, pachymorph growth pattern that forms a slow-growing clump radiating from the initial plant.
Rhizome Propagation
Division
•Cut into pieces containing at least one bud
•Pip: section of rhizome with roots and terminal bud (leaf or flower).
Pseudobulbs
A specialized storage structure above-ground produced by many orchid species, consisting of an enlarged, fleshy section of the stem made up of one to several nodes.
“false bulb”
Propagation of Pseudobulbs
•Offshoots
•Division
Performed during dormancy
Must contain four to five pseudobulbs in the new section
•Back bulbs
Do not have foliage
Removed from plant, placed in rooting medium, new shoots develop
•Green bulbs
Pseudobulbs with leaves treated with IBA
Tissue culture
Propagating plants using a small piece of tissue
Micropropagation
An asexual propagation method in which plants are manipulated on a cellular level, causing them to duplicate themselves repeatedly and rapidly. Can quickly grow many plants from little plant material.
Hstory of micropropation
•1838: Schleiden and Schwan— “Cell theory” and totipotency
•1902: Haberlandt— attempted in vitro growth of plant cells using hydroponic nutrient solution, sucrose and asparagine. Cells only lived 20 days
•1922: first organ cultures developed, adding yeast extracts
•1934: P.R. White—Developed new growth medium
•Also containing amino acids, vitamins
•1939: Gautheret, White, and Nobecourt—independently used the newly isolated growth hormone, auxin
•1946: Ball—First entire plant via tissue culture
•1948: Skoog and colleagues—understood relationship between Cytokinin and Auxin
•1962: Murashige and Skoog—First standardized artificial growth medium
Low auxin/ high cytokinin
shoots
Low cytokinin/ high auxin
roots
Moderate or unbalanced auxin to cytokinin ratio
Callus
Growth medium
Substance containing nutrients and hormones used for plant growth.
Explant
any part of a plant taken out and grown in a test tube or In vitro, under aseptic conditions in special nutrient media.
Totipotency
the plants have a capacity to generate a whole plant from any explant.
Explant requirments
•Light
•Nutrition
•Hormones
•Moisture
•Appropriate temperature
•Aseptic environment
Maintaining an Aseptic Environment
•Laminar flow hood
•Test tubes and petri dishes
•Artificial light supply
•Growth medium
•Isopropyl alcohol solution (70%)
•Bleach solution (10%)
Forceps, scalpel, plastic tape
Growth media
•Nutrients
•Growth regulators
•Water
•Sterile
•Liquid or gel
•Grow and sustain explant in vitro
Seed Culture
Seeds may be cultured in vitro to generate seedlings or plants. It is the best method for raising the sterile seedling.
Embryo Culture
•Embryo culture is the sterile isolation and growth of an immature or mature embryo in invitro with the goal of obtaining a viable plant.
•In some plants seed dormancy may be due to chemical inhibitors or mechanical resistance, structures covering the embryo. Excision of embryos and culturing them in nutrient media help in developing viable seedlings
Meristem culture
•The apical meristem of shoots can be cultured to get the virus-free plants.
•The size of explant may vary from 1.0-5.0 mm long meristem tip
• Often used to produce virus-free bananas
•Rapid multiplication of strawberries, chrysanthemum, African violets
Callus Culture
•Callus: un-organized dedifferentiated mass of cells arising from any kind of explant under in vitro cultural conditions.
•The cells in callus are parenchymatous in nature but may or may not be homogenous mass of cells.
•After callus induction it can be sub-cultured regularly with appropriate new medium for growth and maintenance.
•Carrot, potato, tobacco
Anther or Pollen Culture
•This technique involves culturing anthers to produce haploid plants, which have only one set of chromosomes.
•First established by Guha and Maheswari (1964, 1966) in Datura.
•Haploid plants are valuable for genetic research because they simplify the study of gene expression and inheritance
•Treatments can create diploid plants that are genetically uniform.
•Commercial breeding of tobacco since 1967
Stages of Micropropagationgation
•Stage 0: Selection and Cultivation of Stock Plants
•Stage 1: Initiation or Establishment
•Stage 2: Multiplication
•Stage 3: Rooting
•Stage 4: Acclimatization
Stage 0: Selection and Cultivation of Stock Plants
•Meticulous cultivation of stock plants
•Ensure disease free
•No insects
•Stage 0 limits and prevents contamination
Stage 1: Initiation or Establishment
•Aseptic environment
•Explant must be sterilized
•Explant transferred to in vitro culture
•Explant material sources
•Single cells from plants
•Small pieces of plant tissues
•Apical meristem
Stage 2: Multiplication
•Focuses on the multiplication and shoot growth of the plantlets
•Explants transferred to multiplication medium
•Must prevent contamination
•Medium composition
•Gel containing vitamins, sugars, and a plant growth regulator
Plantlets
•ready for transfer to stage 3 when
•Shoots and leaves present
•Rich in green pigment
•Do not have any roots
Stage 3: Rooting
•Plantlets transferred to rooting medium which contains
•Nutrients
•Growth regulators
•Sterile
•Roots develop below medium surface
•Root presence indicates transplant readiness
•Time required varies weeks to months
Stage 4: Acclimatization
•Acclimatization: the gradual exposure of plants to different environmental conditions; also known as hardening-off.
•Plantlets transferred to a sterile potting medium
•Acclimatization occurs
Roots washed to remove stage 3 medium
•Rooted plantlets need to be acclimatized before they can resume growth outdoors or in the greenhouse.
•(a) An enclosed mist system where the sidewalls roll up to gradually reduce humidity and raise light levels. (b) A high humidity poly tent covers an entire greenhouse bench for acclimatizing plants. (c) Poly covers on individual flats. (d) The polyethylene film is cut to provide gradual acclimatization. (e) Fully acclimatized purple coneflower (Echinacea).
Phase Potential
Thin-layer explants of epidermal tissue showing organ initiation potential relative to location on the mother plant where the explant was taken.
Supply and Demand
•Micropropagated materials are costly
•Plant must be in demand
•Must be desired in large quantities
Plants commonly micropropagated
Orchids, bananas, potataoes, strawberries, African violets and spider plants
Advantages of micrpropagation
•Uniformity of clones
•Ability to grow large numbers
•Produce plants when usually not compatible or difficult to propagate
•Vast quantities of clones from one plant
•Ability to produce pest-free plants
•Aid in conservation and replication of rare or endangered plants
Disadvantages of Micropropagation
•Requires expensive and sophisticated facilities, trained personnel, & specialize techniques.
•High labor costs.
•A high-volume distribution system, or adequate storage facilities to stockpile products, is required.
•Pathogen contamination or insect infestation can cause high losses in a short time.
•Variability and production of off-type individuals can be a risk.
Decrease this risk by careful roguing, field testing and continuing research
•More companies fail for economic reasons than because of an inability to produce micropropagated plants.
Bioenginering
The artificial manipulation, modification, and recombination of DNA or other nucleic acid molecules in order to modify an organism or population of organisms
GMO (genetically modified organism)
•Organism whose genome has been engineered in the laboratory to favor the expression of desired physiological traits or the generation of desired biological products
Difference between gene-edited and transgenic
•Both are genetically modified
•Gene-edited uses or changes host DNA sequencing
•Transgenic is taking foreign DNA and introducing it
Methods of bioengineering
•Gene cloning/ Transformation
•Agrobacterium-Mediated Transformation
•Gene Gun (Particle Bombardment)
•CRISPR-Cas9 Gene Editing
•RNA Interference
Gene cloning/ Transformation
•Goal: Isolate and copy a gene of interest.
•Process:
•The gene is identified in a donor organism.
•It’s cut out using restriction enzymes.
•It’s inserted into a plasmid (a circular piece of DNA).
•The plasmid is introduced into a host bacterium which replicates it, producing many copies.
Transformation
•Once cloned, the gene must be inserted into the target organism.
Bt Crops
Genetically modified plants engineered to contain proteins toxic to certain insect pests, derived from the bacterium Bacillus thuringiensis (Bt)
Roundup Ready Crops
Resistant crops to glyphosate
Agrobacterium-Mediated Transformation
Agrobacterium tumefaciens is a soil bacterium that naturally transfers DNA into plant cells, causing crown gall disease.
Amflora Potato (BASF, EU)
Produces pure amylopectin starch, ideal for industrial uses like paper and textiles
Flavr Savr Tomato
First genetically modified food crop to be commercialized (approved in 1994)
Gene Gun (Particle Bombardment)
How it works:
•Microscopic gold or tungsten particles are coated with the desired DNA.
•These are shot at high velocity into plant cells or tissues using a burst of helium gas.
•Some DNA makes it into the nucleus and integrates into the plant’s genome.
•Transformed cells are selected and regenerated.
Golden Rice
Biofortified with pro-vitamin A (β-carotene)
Starlink Corn
Insect resistance using a variant of the Bt toxin
Waxy Corn
Produces 100% amylopectin starch ideal for food processing, adhesives, and textile manufacturing
CRISPR-Cas9 Gene Editing
•CRISPR stands for Clustered Regularly Interspaced Short Palindromic Repeats.
•Cas9 is an enzyme that acts like molecular scissors.
•A guide RNA (gRNA) is designed to match a specific DNA sequence.
•Cas9 + gRNA complex binds to the target sequence in the genome.
•Cas9 cuts the DNA at that location.
•The cell tries to repair the break:
•Non-Homologous End Joining (NHEJ): An error-prone process that may knock out a gene.
•Homology-Directed Repair (HDR): A more precise repair process, especially if a DNA template is provided to insert new genes.

CRISPR Tomato (Sanatech Seed, Japan)
Increased GABA content, a compound linked to stress reduction and lower blood pressure
CRISPR Lettuce (in research)
Improved shelf life, reduced browning or bolting delay (early flowering in warm temps)
RNA Interference
•RNAi uses double-stranded RNA (dsRNA) molecules that are complementary to the mRNA of a target gene.
•Inside the cell, these dsRNA molecules are chopped into short fragments by an enzyme called Dicer.
•These fragments are loaded into the RNA-induced silencing complex (RISC), which uses them to find and degrade the corresponding mRNA.
•This blocks the production of the target protein.
Synthetic Biology
Goes beyond simply adding a gene — this involves designing entire metabolic pathways using genes from multiple organisms or even building new genetic systems from scratch.
Current Market GMOs
•Cotton
•Corn
•Soybean
•Canola
•Alfalfa
Sugar Beets
•Papaya
•Squash
•Potato
•Apples
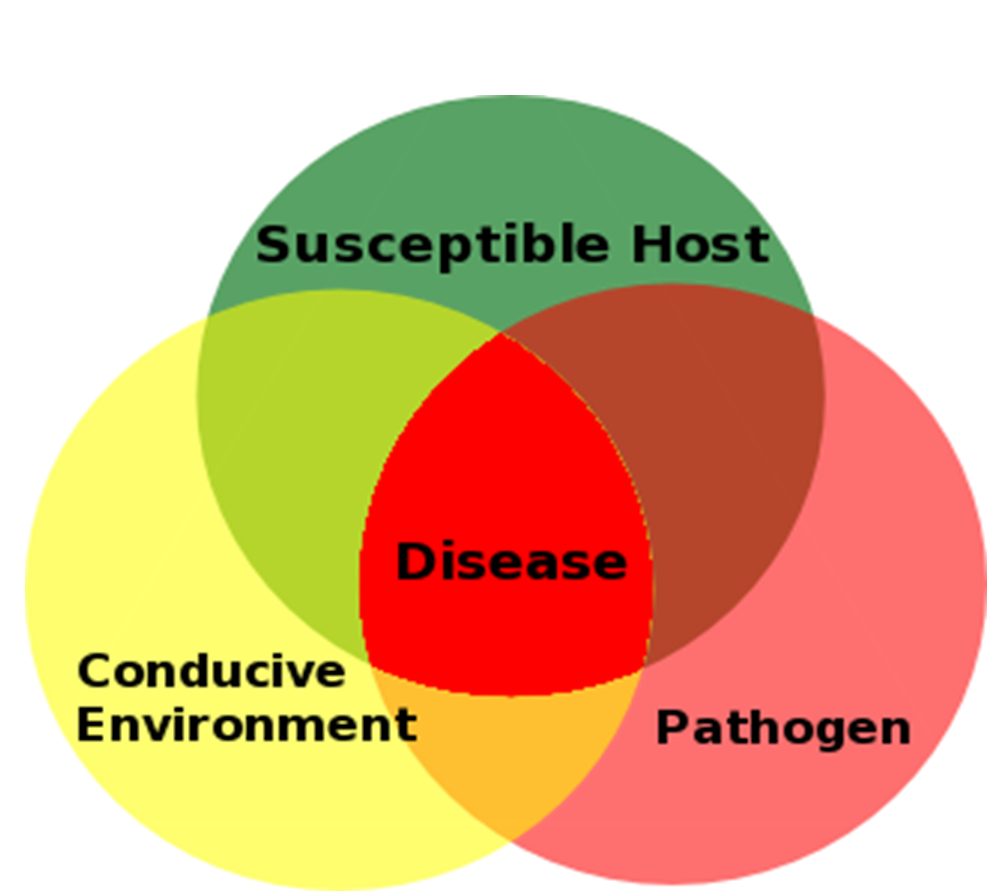
Disease Triangle
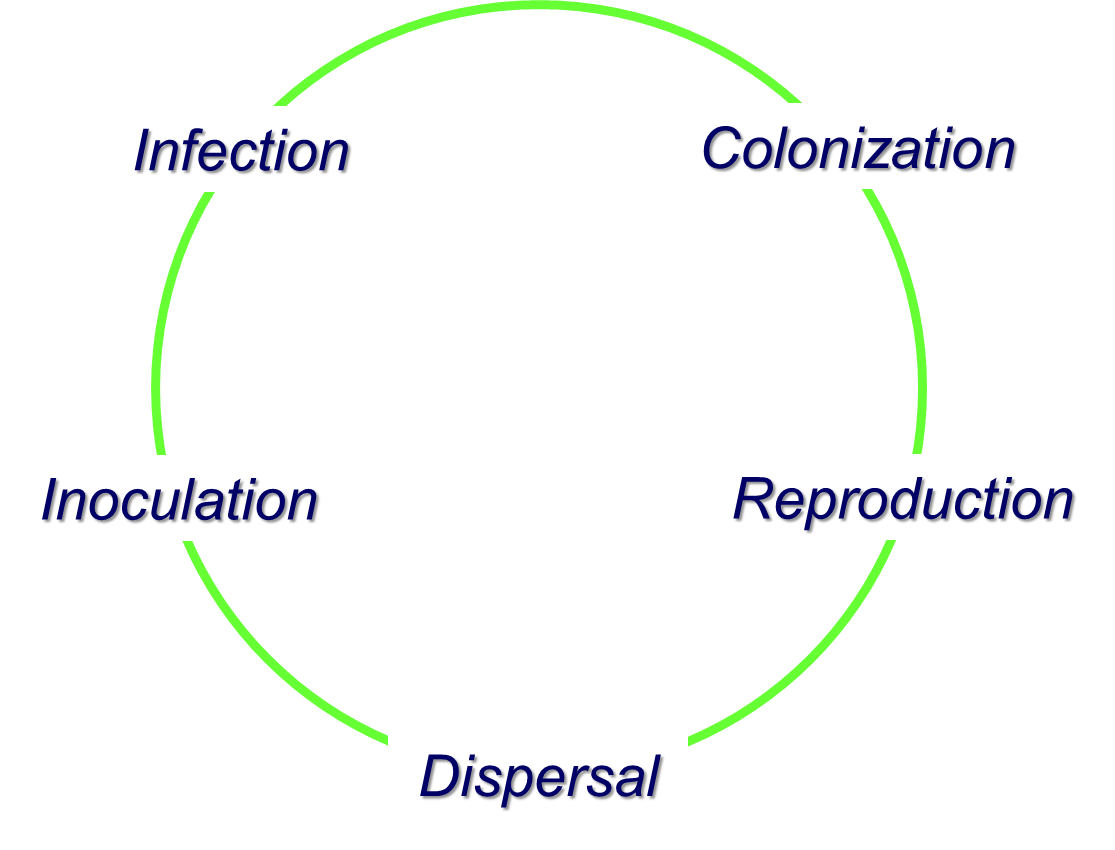
Disease Cycle
Symptoms
the visual effects produced by pathogens or pests
Signs
physical evidence of the pathogens or pests