the histaminergic system and antihistamines
1/27
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
28 Terms
what are autocoids?
LOCAL HORMONES (short acting endogenous mediators usually acting as part of an inflammatory response - substances are released locally in the body
- some are protective
- some are related to the development of disease
what are examples of autocoids?
• Bradykinin
• Eicosanoids
- Prostaglandins, thromboxanes, leukotrienes
• Histamine
• Kallidin
• Platelet activating factor (PAF)
• Serotonin
where is histamine present?
• mast cells - which are abundant in the skin, GI, and
the respiratory tract. histamine sits and a signal causes degradation for release
• basophils in the blood
• some neurons in the CNS and PNS (act as
neurotransmitter)
what are the store cells of histamine?
- ECL and G cell - GI tract
- mast cell
- basophil
- neuron
what is the common effects of histamine when it reaches its target cell?
- acid secretion
- mucosal protection
- fluid transport
- neurotransmission
- visceral sensitivity
- motility
- inflammation
- allergy
- tumor growth
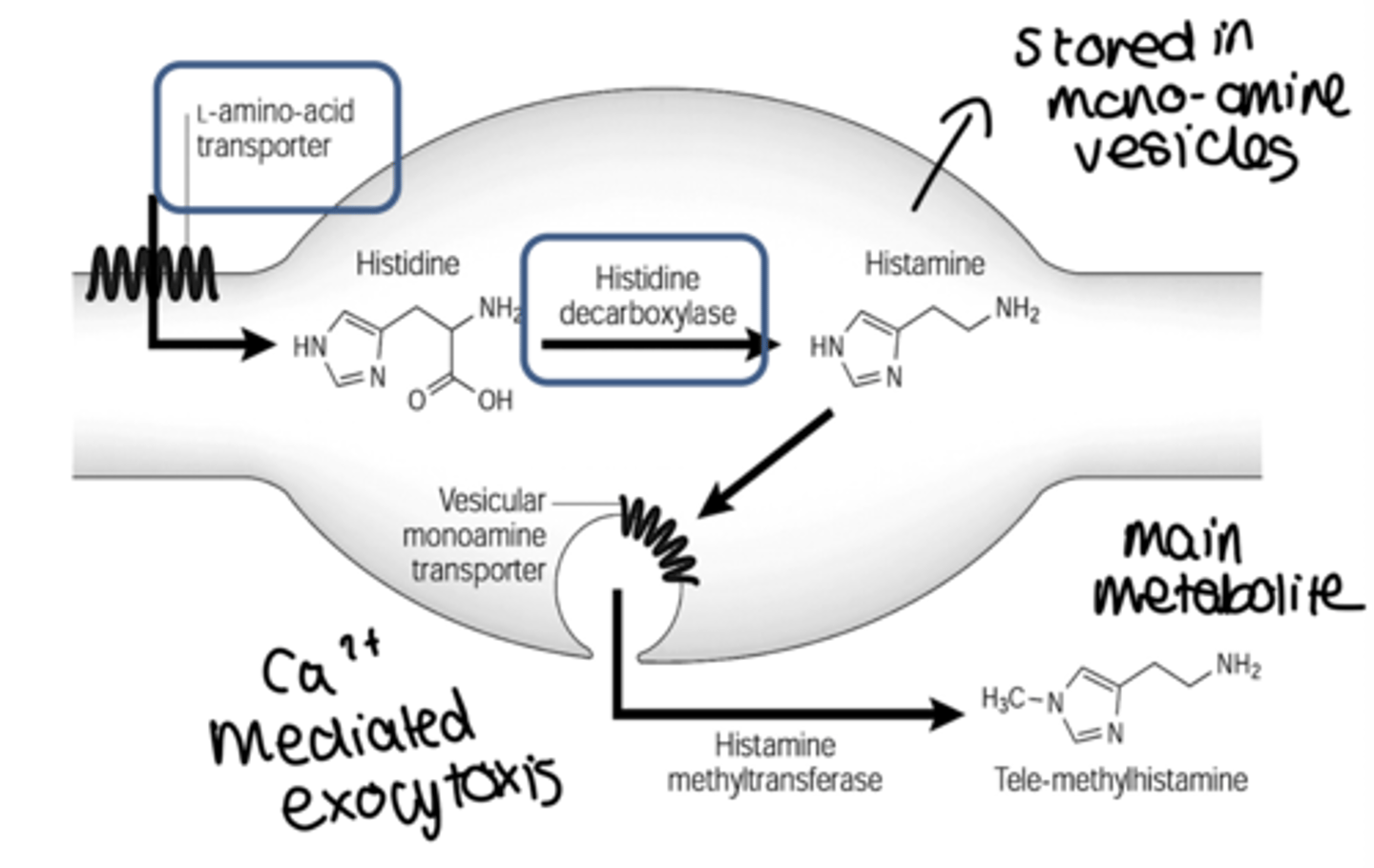
how is histamine synthesied and metabolised?
• Synthesized by decarboxylation (HDC) from an amino acid
precursor, histidine
• Stored in granules in certain cells (mast cells, basophils,
enterocytes)
• Released in response to certain stimuli (Ca dependent
exocytosis)
• Eliminated by oxidative deamination and/or transmethylation
what are the 4 histamine receptors, what do they control?
H1: Allergy
H2: Acid Reflux
H3: CNS/PNS
H4: Inflammation and itch
where are H1 receptors present? what are their effects?
Smooth muscle, endothelium, CNS.
- Bronchoconstriction
- vasodilation
- separation of endothelial cells
- pain and itching
- allergic rhinitis
- motion sickness.
where are H2 receptors present? what are their effects?
gastric parietal cell, vascular smooth muscle cells, basophils.
- Regulate gastric acid secretion
- vasodilation
- inhibition of IgE dependent degranulation.
where are H3 receptors present? what are their effects?
CNS cells, and some in peripheral NS
- Presynaptic feedback inhibition of histamine synthesis and release
- control release of DA, GABA, ACh, 5-HT & NE.
where are H4 receptors present? what are their effects?
Highly expressed in bone marrow and white blood cells.
- Mediate mast cell chemotaxis.
what family are the histamine receptors part of?
G-protein coupled
ligand binds for a response
what is the G protein pairing in H1 receptors?
Gq coupled to Phospholipase C
what is the G protein pairing in H2 receptors?
Gs coupled to Adenylyl Cyclase (AC); increase cAMP
what is the G protein pairing in H3 receptors?
Gi/o coupled to AC, also to K- channels and reduce Ca influx, inhibit presynaptic neurotransmitter release
what is the G protein pairing in H4 receptors?
available data consistent with coupling to Gi/o in mast cells,
as well as eosinophils, that can trigger calcium mobilization - mast cell chemotaxis
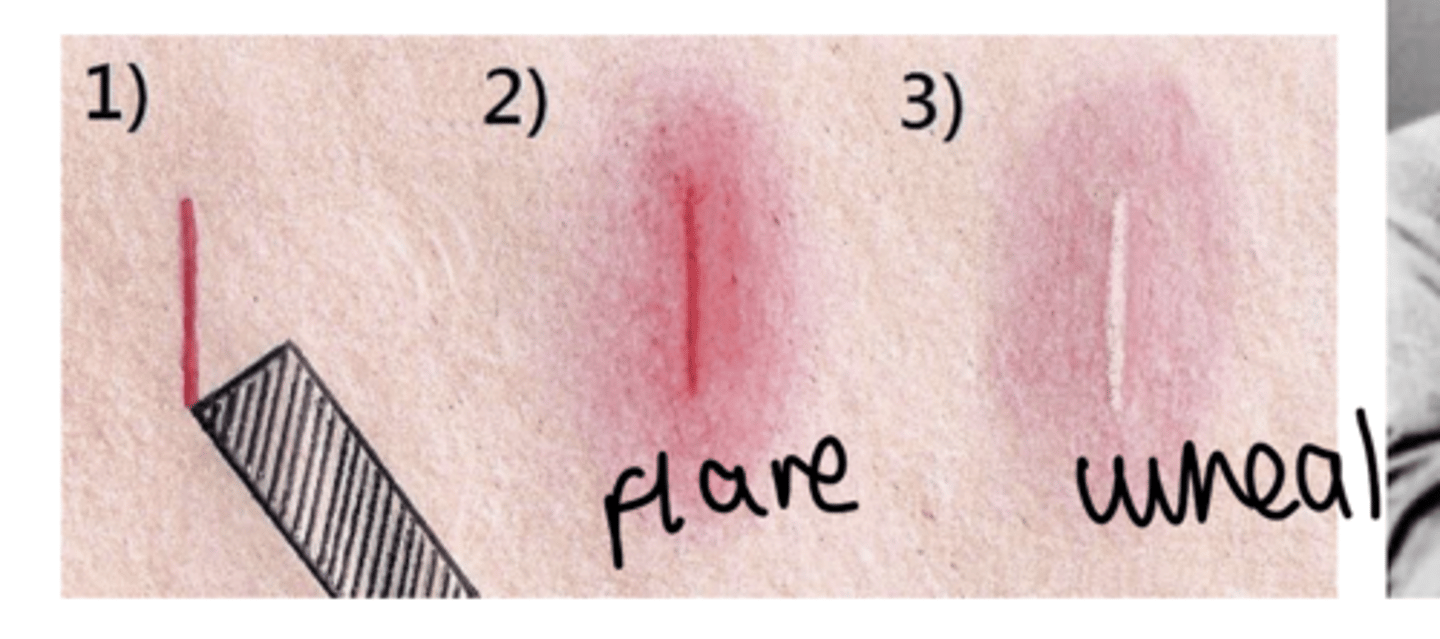
what is the intradermal triple response of histamine?
• Red spot: (few mm) in seconds: direct vasodilation effect , H1 receptor
mediated;
• Flare: (1cm beyond site): axonal reflexes, indirect vasodilation, and
itching, H1 receptor mediated;
• Wheal: (1-2 min) same area as original spot, edema due to increased
capillary permeability, H1 receptor mediated.
what are the effects of histamine on the human heart?
- affects both cardiac contractility and electrical events directly.
- increases the force of contraction of both atrial and ventricular muscle
- promoting the influx of Ca2+, and it speeds
heart rate by hastening diastolic depolarization in the sinoatrial (SA)
node.
- in anaphalaxis, increased HR to combat a decrease in BP, which is driven by histamine
what are the effects of histamine receptors on the human lung?
- H1- bronchoconstriction, increased mucus viscosity
- H2- slight bronchodilation, increased mucus secretion
- H1- stimulation of vagal sensory nerve endings: cough
what compounds promote histamine release?
• Therapeutic:
morphine (itch) or “red neck syndrome” induced by vancomycin (ABx)
• Experimental:
compound 48/80
• Unknown:
cause of hives is rarely determined - but more identifiable by reoccurance
• Antigen-antibody reactions
what is anaphylaxis?
Histamine induced reactions range from mild allergic symptoms to anaphylactic shock
- Type I allergic response - (immediate hypersensitivity reaction)
- Mediated by IgE antibodies
- IgE binds to receptors on mast cells and basophils
- Fab portion of antibody binds antigen and causes (moderate to massive) release of: histamine, leukotrienes, prostaglandins
what is the effects of anaphylaxis?
• Decreased blood pressure
• Decreased cardiac output
• Bronchoconstriction and increased pulmonary secretions
• Pruritus (itching)
what is the treatment of anaphylaxis?
Treatment: Epinephrine - not initially antihistamines
(epinephrine is a physiological antagonist of histamine, not a
pharmacological antagonist)
(alpha-1 vasoconstriction, beta-1 increased HR,
beta-2 bronchodilation)
ADRENERGIC receptors are utilised instead of histamine receptors to oppose effect of histamine without acting upon the same receptor
what are the 3 mechanistically different approaches to minimise histamine reactions?
• Physiological antagonism (e.g. adrenaline, ephinerpherine)
• Inhibit the release of histamine (e.g. cromolyn) - prevents degredation of mast cells
• Pharmacological antagonism (antihistamines)
what are the available histamine related drugs?
- Mast Cell Stabilizers (Cromolyn)
- H1 Receptor Antagonists (1st and 2nd generation)
- H2 Receptor Antagonists (Ranitidine, Cimetidine) - regulates secretion of stomach acid
- H3 Receptor Antagonists (Pitolisant) - increases histamine in brain
- H3 and H4 Receptor Agonist and Antagonists (potential newdrugs being developed)
what is the pharmacokinetics of 2nd gen H1RA's?
- well absorbed and are excreted mainly unmetabolized form.
• primarily excreted in the urine
• they induce CYP450 liver enzymes
what are H3RA's used for?
Pitolisant
• Enhances the activity of brain histaminergic neurons.
• Designed to promote wakefulness and so reduce excessive daytime sleepiness (EDS), whilst also resulting in a significant reduction in cataplexy.
• Helps reduce the symptoms of idiopathic hypersomnia.
- used for narcoleptics
- binds to H3 like an agonist, but behaves as an antagonist
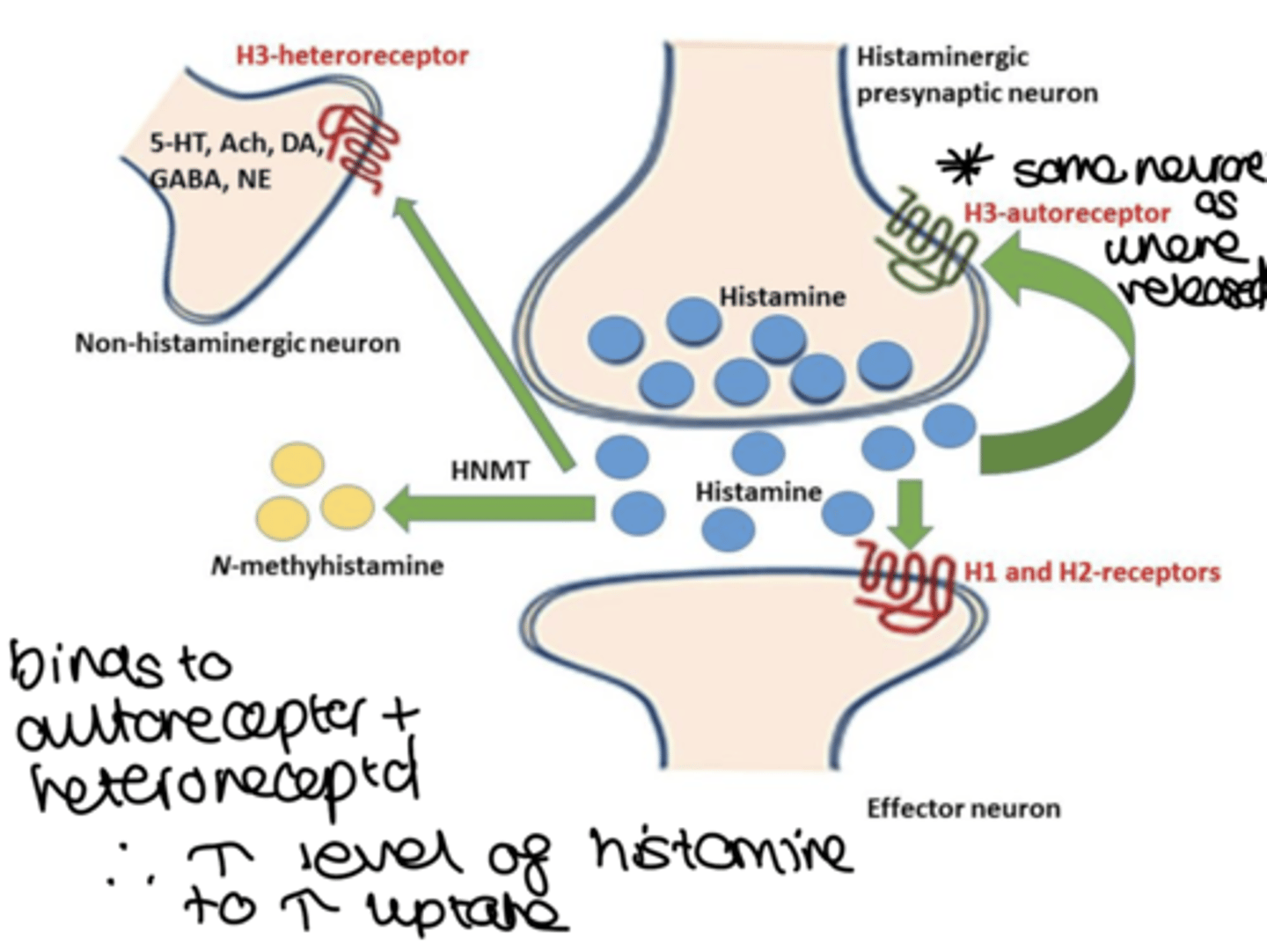
how is a H3RA considered an inverse agonist?
- they bind to the H3 receptor and produce the opposite effect of what the receptor would typically induce when activated by histamine
- when H3 is activated by histamine, it inhibits the release of neurotransmitters
- so when histamine binds to the H3 receptor, it reduces the release of other neurotransmitters like dopamine, norepinephrine, and acetylcholine.
- when H3 receptor antagonist binds to the receptor, it prevents histamine from activating, but since the H3 receptor has a constitutive activity (it can be active even without histamine), the antagonist not only blocks histamine's effect but also reduces this baseline activity. This reduction in constitutive activity leads to an increase in the release of neurotransmitters, which is why these antagonists are classified as inverse agonists
- so they block the action of histamine and decrease the receptors constitutive activity, leading to increased neurotransmitter release.