Iverson CH320M Final Flashcards
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
14 Terms
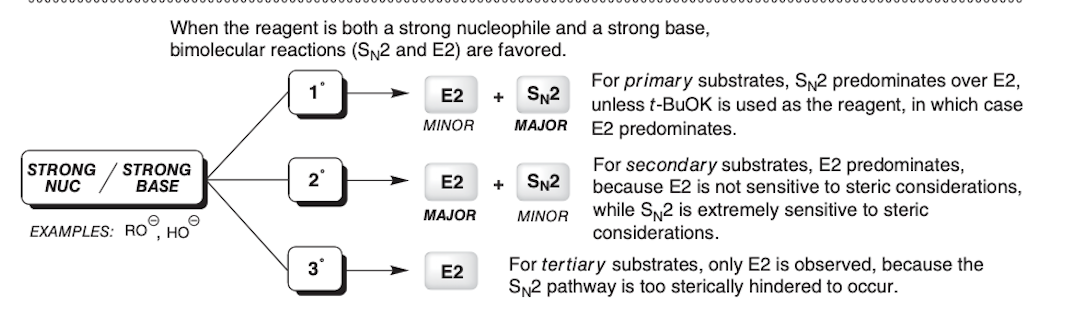
For SN2 reactions __________ are most reactive whereas in SN1 reactions __________ are most reactive
Primary, Tertiary
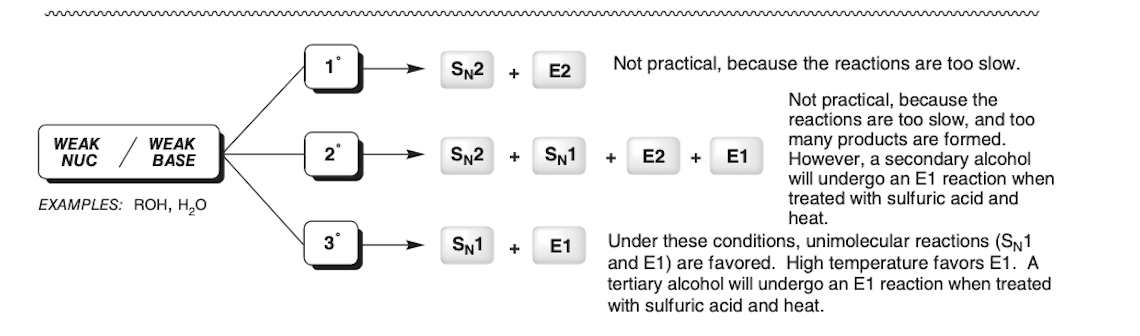
Whats the difference in the first step of an SN1 reaction compared to the first step of an SN2 reaction?
The first step of an SN2 reaction is the nucleophile attacking the electrophile while the first step of an SN1 reaction is the leaving group leaving
In SN1 reactions the __________ of the __________ is most important
stability, carbocation
1° and 2° substrates are more likely to be __________
SN2
Good leaving groups are often __________
Conjugate Bases
__________ speed up the rate of SN2 reactions
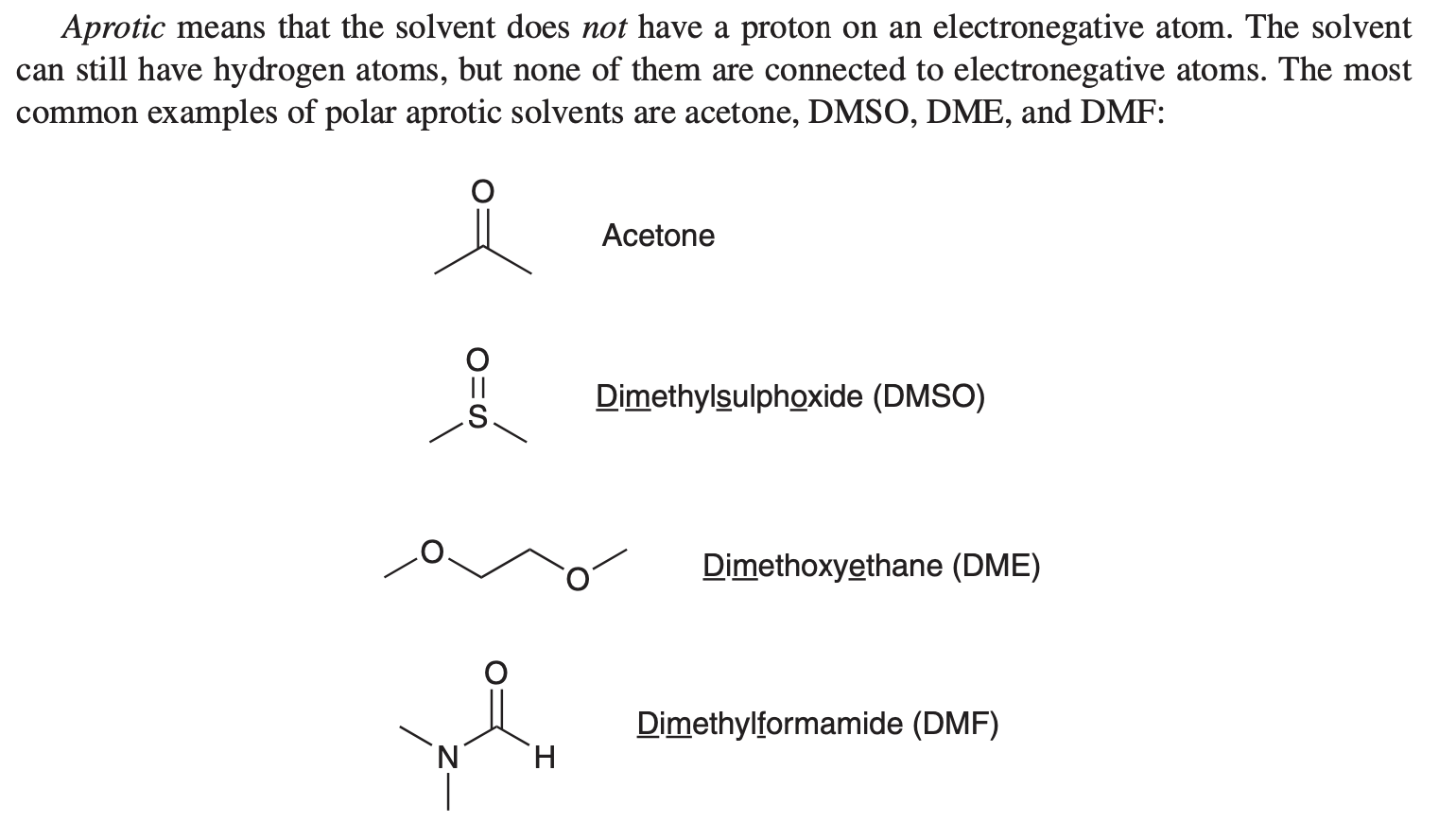
polar aprotic solvents

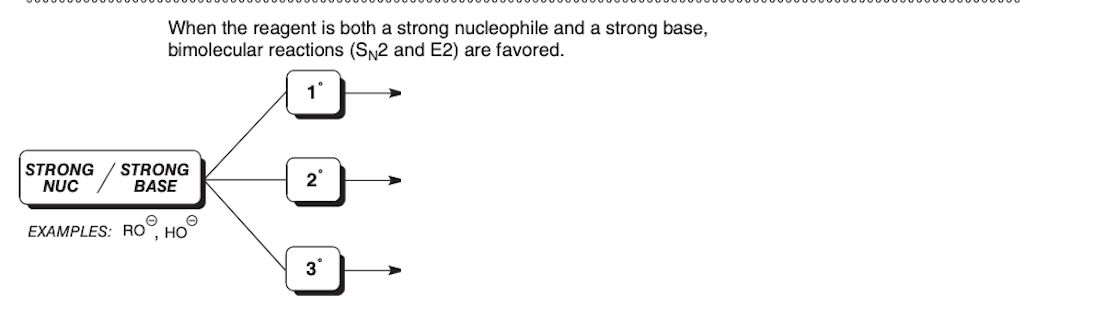

Cl-
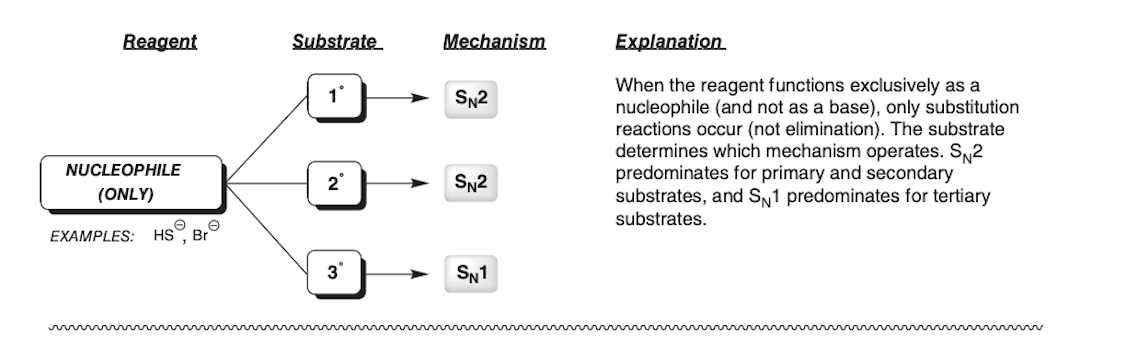
Nucleophile only
Br-
Nucleophile only
In order to identify the mechanism of a reaction first __________ and then __________
identify the function of the reagent, identify the substrate
If you have a nucleophile who’s conjugate acid is strong then its a __________
weak base/strong nucleophile