Biochem Midterm Review
0.0(0)
Card Sorting
1/165
Earn XP
Description and Tags
Last updated 8:25 PM on 4/15/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
166 Terms
1
New cards
What must amino acids have?
\-amino group
\-carboxyl group
\-hydrogen
\-side chain
\-carboxyl group
\-hydrogen
\-side chain
2
New cards
Is the L or D configuration used to make proteins?
\-L is used
3
New cards
How are amino bonds created?
\-condensation at the c- terminus and n-terminus of the two amino acids
4
New cards
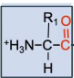
What makes up a residue?
\-amino acid
\-n-terminus
\-c-terminus
\-n-terminus
\-c-terminus
5
New cards
What makes up a backbone?
\-everything but the side chains
6
New cards
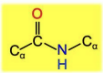
Identify where the omega, psi and phy bonds are and some info on them
\-no rotation in omega
\-alpha carbon is usually trans
\-C-N bond is a partial double bond because of resonance
\-psi is between alpha carbon and carboxyl
\-phi is between alpha carbon and amino nitrogen
\-rotation is possible with psi and phi bonds but remember steric hinderance
\-alpha carbon is usually trans
\-C-N bond is a partial double bond because of resonance
\-psi is between alpha carbon and carboxyl
\-phi is between alpha carbon and amino nitrogen
\-rotation is possible with psi and phi bonds but remember steric hinderance
7
New cards
Types of Non-covalent interactions
\-charge-charge
\-hydrogen bond
\-van der Waals
\-hydrogen bond
\-van der Waals
8
New cards
2 Secondary Structures that arise from backbone H-bonding in the chain
\-alpha helix
\-beta sheet
\-beta sheet
9
New cards
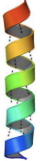
Alpha Helixes
\-h-bonds are co-linear with chain reaction
\-residue n provides backbone carboxyl(H-bond acceptor)
\-residue n+4 provides amino(H-bond donor)
\-there are 3.6 residues perf turn
\-residue n provides backbone carboxyl(H-bond acceptor)
\-residue n+4 provides amino(H-bond donor)
\-there are 3.6 residues perf turn
10
New cards
Beta Sheets
\-each strand is nearly flat
\-H-bonds are nearly right angles to the direction of the chain
\-will always have at least 2 strands
\-can either be parallel(usually flatter) or antiparallel
\-antiparallel have a slight twist
\-H-bonds are nearly right angles to the direction of the chain
\-will always have at least 2 strands
\-can either be parallel(usually flatter) or antiparallel
\-antiparallel have a slight twist
11
New cards
How does water affect proteins
\-proteins are either polar or non-polar
\-non-polar residues tend to be found inside when a protein is folded
\-polar will be found on the outside when a protein is folded
\-van der Waal interactions will occur between closely packed atoms
\-non-polar residues tend to be found inside when a protein is folded
\-polar will be found on the outside when a protein is folded
\-van der Waal interactions will occur between closely packed atoms
12
New cards
Aliphatic Side chains
\-they only have hydrogen and carbon in the chain
\-they are not aromatic
\-nonpolar
\-they are not aromatic
\-nonpolar
13
New cards
Polar Uncharged side chains
\-either have an alcohol, amide or thiol
\-no positive or negative charge
\-no aromatics
\-cysteine has strong disulfide bond
\-no positive or negative charge
\-no aromatics
\-cysteine has strong disulfide bond
14
New cards
Fibrous Proteins
\-long extended structure
\-composed of single secondary structure
\-tend to have repeat sequences
\-tend to use few types of residue
\-composed of single secondary structure
\-tend to have repeat sequences
\-tend to use few types of residue
15
New cards
Alpha Keratin
\-fibrous protein composed of alpha helixes
\-runs parallel
\-has disulfide bond
\-common residues are Ser, Cys, Gln and Glu
\-runs parallel
\-has disulfide bond
\-common residues are Ser, Cys, Gln and Glu
16
New cards
Fibrion
\-fibrous protein composed of beta sheets
\-van der Waals interaction btwn sheets make silk felxible
\-enriched in Gly, Ala, and Ser
\-Gly alternates
\-van der Waals interaction btwn sheets make silk felxible
\-enriched in Gly, Ala, and Ser
\-Gly alternates
17
New cards
Collagen
\-composed of a triple helix
\-rich in Gly, Ala, and Pro
\-long protein chains
\-3 chains wrap around each other
\-rich in Gly, Ala, and Pro
\-long protein chains
\-3 chains wrap around each other
18
New cards
What are consequences of Proline?
\-no amide hydrogen
\-makes no backbone
\-can’t be in alpha helix or beta sheet
\-makes no backbone
\-can’t be in alpha helix or beta sheet
19
New cards
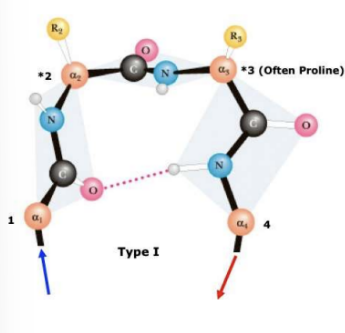
Type 1 Beta sheet Turns
\-have cis geometry about peptide bonds between residues 2 and 3
\-proline favours this geometry(depends on direction of protein chain)
\-proline favours this geometry(depends on direction of protein chain)
20
New cards
Type 2 Beta sheet Turns
\-usual trans geometry in central peptide bond of residues 2 & 3
\-residue at 3 must be small and is usually a glycine
\-residue at 3 must be small and is usually a glycine
21
New cards
Which of the amino acids is not a chiral molecule?
\-glycine
22
New cards
Acidic Residues
\-have net negative charge at pH 7
\-uncharged in protonated state and -vely charged in deprotonated state
\-have carboxylic acid group in side chain
\-uncharged in protonated state and -vely charged in deprotonated state
\-have carboxylic acid group in side chain
23
New cards
Basic Residues
\-have net positive charge at pH 7
\-positively charged in protonated state and uncharged in deprotonated state
\-functional groups include a primary amine, guanidinium group and basic imidazole ring
\-positively charged in protonated state and uncharged in deprotonated state
\-functional groups include a primary amine, guanidinium group and basic imidazole ring
24
New cards
What interaction is between Acidic and Basic Side Chains
\-charge-charge interactions
\-opposites attract and like charges repel
\-opposites attract and like charges repel
25
New cards
Aromatic Residues
\-non-polar
\-very stable
\-some have one ring(Phe, Tyr) and others have two(Trp)
\-absorb light in ultra-violet region
\-very stable
\-some have one ring(Phe, Tyr) and others have two(Trp)
\-absorb light in ultra-violet region
26
New cards
Special Methionine
\-sulfur-containing nonpolar residue
\-properties resemble nonpolar aliphatic residues
\-the first to be translated in protein chain
\-properties resemble nonpolar aliphatic residues
\-the first to be translated in protein chain
27
New cards
How do Globular Proteins differ from each other?
\-sequence
\-function
\-fold
\-function
\-fold
28
New cards
What are the levels of Protein Structures?
\-primary(the sequence of residues)
\-secondary(backbone H-bonding, helixes and sheets)
\-Tertiary(folding of secondary structure)
\-quaternary(interactions between two or more protein subunits)
\-secondary(backbone H-bonding, helixes and sheets)
\-Tertiary(folding of secondary structure)
\-quaternary(interactions between two or more protein subunits)
29
New cards
Patterns of Main-Chain Folding
\-classify by dominant secondary structures
\-composed of more than one domain(\~150-200 residues)
\-domains can be composed of motifs(secondary structures adjacent in sequence)
\-most common motifs are Helix Hairpin, Beta hairpin and Beta-Alpha hairpin
\-composed of more than one domain(\~150-200 residues)
\-domains can be composed of motifs(secondary structures adjacent in sequence)
\-most common motifs are Helix Hairpin, Beta hairpin and Beta-Alpha hairpin
30
New cards
Why do proteins fold?
\-have a very specific solid confirmation
\-depends of steric clashes
\-depends of steric clashes
31
New cards
How do you denature a protein?
\-increase temp.
\-change pH to extremely basic or acidic
\-add chaotropic molecules(competition to H-bonding in backbone)
\-is reversable
\-change pH to extremely basic or acidic
\-add chaotropic molecules(competition to H-bonding in backbone)
\-is reversable
32
New cards
How to reverse denaturing a protein?
\-lower the temp.
\-restore pH
\-remove chaotrope
\-restore pH
\-remove chaotrope
33
New cards
What conditions of G = H -TS favour folding?
\-negative G
\-negative H
\-positive S
\-negative H
\-positive S
34
New cards
Which Thermodynamics factor do we consider regarding folding?
\-conformational entropy(favours unfolded state)
\-charge-charge(favours folding)
\-internal H bonds(favours folding)
\-van der Waals interaction(favours folding)
\-hydrophobic effect(favours folding)
\-charge-charge(favours folding)
\-internal H bonds(favours folding)
\-van der Waals interaction(favours folding)
\-hydrophobic effect(favours folding)
35
New cards
What are ways to stabilize Folded State
\-disulfide bond
\-metal ligand(coordinates bonds pin the structure together
\-prosthetic ground binding(cofactor)
\-metal ligand(coordinates bonds pin the structure together
\-prosthetic ground binding(cofactor)
36
New cards
What do Quaternary structures accomplish?
\-increased stability in folded state
\-assembly of large structures
\-a major form of enzyme activity control
\-assembly of large structures
\-a major form of enzyme activity control
37
New cards
What are invariant residue?
\-positions in the aligned sequences where there is an exact amino acid residue match
38
New cards
What are conservative substitutions?
\-positions in aligned sequences where the match is nit exact but similar residues occurs
eg. glutamic acid and aspartic acid
eg. glutamic acid and aspartic acid
39
New cards
What is identity?
\-percentage of invariant residues between the aligned sequences
40
New cards
What is Similarity?
\-percentage of conservative substitutions + invariant residue between the aligned sequences
41
New cards
If proteins have similar folding, do they have similar functions?
\-no, they’ll have common evolutionary origin
\-they will be apart of protein family
\-they will be apart of protein family
42
New cards
Why is Protein Engineering possible?
\-genetic code is universal
\-molecular biologist and organic chemists have provided the tools
\-molecular biologist and organic chemists have provided the tools
43
New cards
What do you need for recombinant protein expression?
\-expression hosts
\-expression vectors
\-enzymes for manipulation of DNA
\-synthetic DNA
\-expression vectors
\-enzymes for manipulation of DNA
\-synthetic DNA
44
New cards
Examples of Expression Hosts
\-E.coli(cheap, high yield, easily scalable, no post transitional processing of eukaryotic proteins)
\-Yeast(cheap, high yield, no post transitional processing of eukaryotic proteins, tough to lyse the cells)
\-Yeast(cheap, high yield, no post transitional processing of eukaryotic proteins, tough to lyse the cells)
45
New cards
Expression Vectors
\-plasmid
\-signals to increase levels of transcription and translation
\-cloning site
\-selectable marker
\-origin of replication
\-signals to increase levels of transcription and translation
\-cloning site
\-selectable marker
\-origin of replication
46
New cards
What are the steps to recombinant Protein expression?
\-obtain target sequence
\-cut with restriction enzymes
\-ligate to expression vector and cut with same enzymes
\-introduce expression vector into host
\-host expresses protein
\-cut with restriction enzymes
\-ligate to expression vector and cut with same enzymes
\-introduce expression vector into host
\-host expresses protein
47
New cards
What can Protein Engineering Do?
\-large-scale production of valuable proteins(medicines)
\-expression of proteins from rare source(ancient proteins from species)
\-creation of tagged proteins for ease of purification and tracking(His6)
\-expression of proteins from rare source(ancient proteins from species)
\-creation of tagged proteins for ease of purification and tracking(His6)
48
New cards
What are antibodies?
\-proteins in the body that bind to antigens
\-all have a common framework
\-diverse
\-all have a common framework
\-diverse
49
New cards
How many light and heavy chains does an antibody have?
\-2 heavy and 2 light
\-light is half the size of heavy
\-four Ig fold in each heavy chain
\-two Ig fold in each light chain
\-light is half the size of heavy
\-four Ig fold in each heavy chain
\-two Ig fold in each light chain
50
New cards
How many disulfide bonds and carbohydrates are there in an antibody?
\-4 disulfide bonds
\-2 carbohydrate addiotions
\-2 carbohydrate addiotions
51
New cards
What is the constant domain and what is the variable domain in an antibody?
\-constant is where these are identical amino acid sequences in all class members
\-variable is where there is variation in the domains and this allows antibodies to bind to different targets
\-variable is where there is variation in the domains and this allows antibodies to bind to different targets
52
New cards
What does the Ig Fold look like?
\-a beta sandwich made up of two stacked beta sheets
\-hairpin structures
\-hairpin structures
53
New cards
What is an Fab fragment?
\-half a heavy chain and a whole light chain
54
New cards
What does the Complementary Determining Region refer to?
\-complementary shape binding
\-van der Waals, charge-charge and H-bond interactions
\-van der Waals, charge-charge and H-bond interactions
55
New cards
What are Epitopes
\-antigenic determinants
\-part of antigen that antibody recognize
\-part of antigen that antibody recognize
56
New cards
What type of applications do antibodies have?
\-significant medical and biotech applications
57
New cards
What is the cycle of Enzyme-Catalyzed Reactions
Substrate → Enzyme-Substrate Complex → Enzyme-Transition State Complex → Enzyme-Product Complex(Product leave after) → Free Enzyme
58
New cards
What is the lock and key model of Enzyme Specificity?
\-enzyme = lock and substrate = key
\-complementary interactions
\-explains specificity but not catalysis
\-complementary interactions
\-explains specificity but not catalysis
59
New cards
What is the Induced Fit Model of Enzyme Specificity and Catalysis?
\-complementary interactions
\-as substrate binds, both enzyme active site and substrate undergo conformational changes
\-as substrate binds, both enzyme active site and substrate undergo conformational changes
60
New cards
Mechanism y Which Enzymes Achieve Rate Acceleration
\-optimizing proximity & orientation of substrates(enzyme puts molecules in correct orientation)
\-substrate and active site distortion(conformational changes)
\-electrostatic catalysis(charges on enzyme stabilize charges TS)
\-metal ion catalysis(metal ions as electrostatic catalysis or to neutral pH
\-general acid/base catalysis(stabilize +ve or -ve charge attacking water)
\-covalent catalysis(hydrolyze peptide bonds)
\-substrate and active site distortion(conformational changes)
\-electrostatic catalysis(charges on enzyme stabilize charges TS)
\-metal ion catalysis(metal ions as electrostatic catalysis or to neutral pH
\-general acid/base catalysis(stabilize +ve or -ve charge attacking water)
\-covalent catalysis(hydrolyze peptide bonds)
61
New cards
What are Serine Proteases?
\-possess a highly reactive serine at active site
\-catalytic triad of residues Ser(deprotonates), His(acts as general base) and Asp(negative charge stabilizes protonated His)
\-catalytic triad of residues Ser(deprotonates), His(acts as general base) and Asp(negative charge stabilizes protonated His)
62
New cards
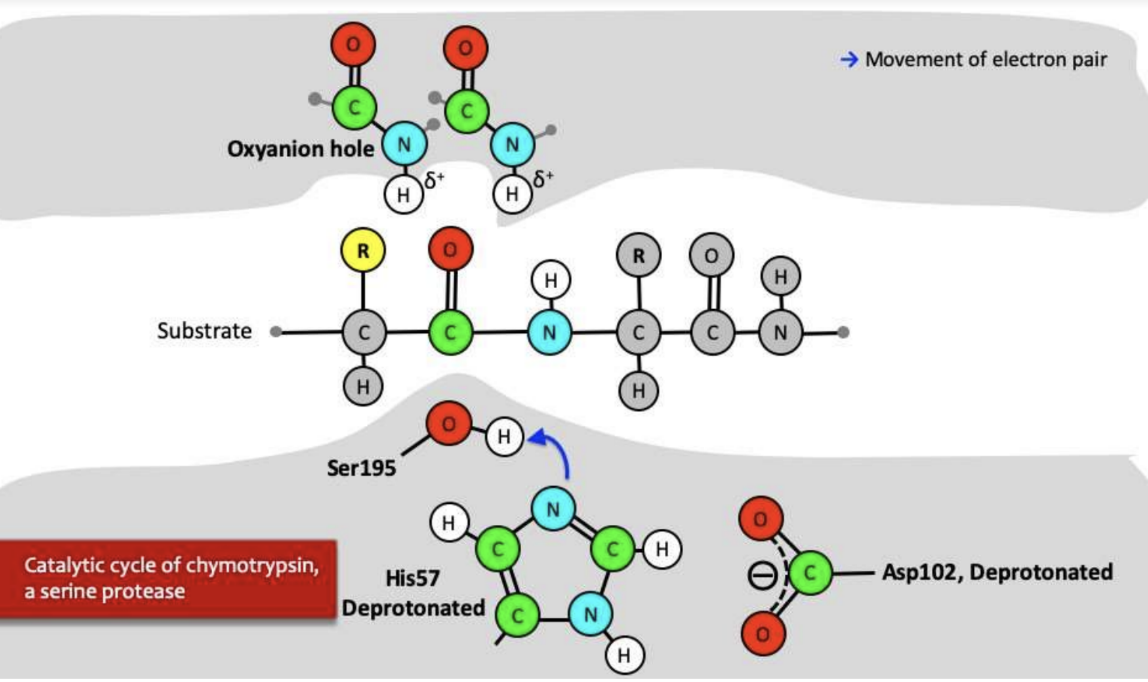
Explain how Serine Protease works?
\-deprotonated His attacks proton on Ser and Ser attacks carbonyl on substrate and makes double bond move to other oxygen, Ser now attached
\-electrons from oxygen move to make double bond and bond from carbon and nitrogen attack protonated His and cleave off and product one is formed
\-deprotonated His attacks water and water attacks carbon of acyl enzyme and double bond moves up to oxygen
\-electrons move from oxygen back down to double bond and Ser attacks proton on protonated His
\-product 2 is now formed, it leaves and process starts all over again
\-electrons from oxygen move to make double bond and bond from carbon and nitrogen attack protonated His and cleave off and product one is formed
\-deprotonated His attacks water and water attacks carbon of acyl enzyme and double bond moves up to oxygen
\-electrons move from oxygen back down to double bond and Ser attacks proton on protonated His
\-product 2 is now formed, it leaves and process starts all over again
63
New cards
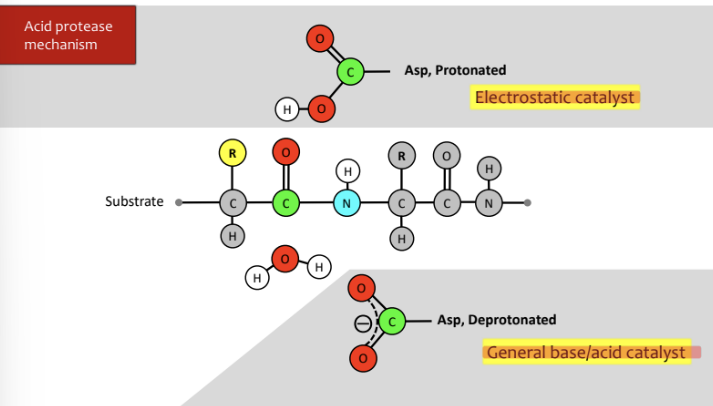
How does Acid Proteases Work?
\-deprotonated Asp attacks water, water attacks carbonyl and moves the double bond with oxygen up to the oxygen
\-electrons from oxygen move to make double bond and bond from carbon and nitrogen attack protonated Asp and cleave off and product is formed
\-electrons from oxygen move to make double bond and bond from carbon and nitrogen attack protonated Asp and cleave off and product is formed
64
New cards
What happens if you inhibit HIV protease? give some examples
\-it stops the whole process
\-eg. Iopinavir and ritonavir
\-eg. Iopinavir and ritonavir
65
New cards
What do Metalloproteases Use?
\-catalytic power of Zn2+
66
New cards
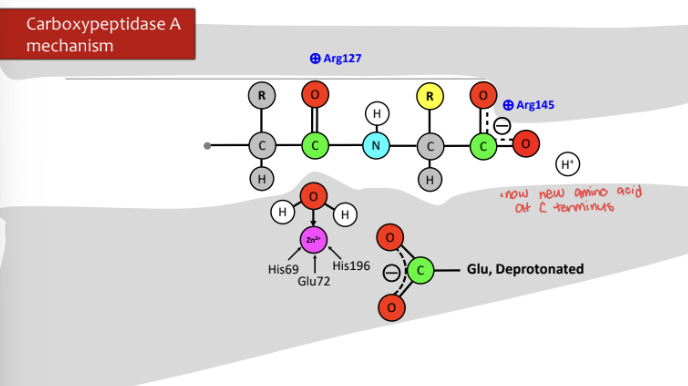
How does Carboxypeptidase A work?
\-deprotonated Glu deprotonates water with Zn2+ and then that group attacks the carbonyl and double bond moves up on oxygen
\-electrons from oxygen move to make double bond and bond from carbon and nitrogen attack protonated Glu and cleave off and amino acid leaves
\-hydroxide Zn2+ molecule gains a proton and cleaves off carbon
\-process starts all over again
\-electrons from oxygen move to make double bond and bond from carbon and nitrogen attack protonated Glu and cleave off and amino acid leaves
\-hydroxide Zn2+ molecule gains a proton and cleaves off carbon
\-process starts all over again
67
New cards
What processes need Enzyme Regulation?
\-digestive enzymes
\-blood clotting enzymes
\-metabolic pathways that provide energy
\-signal transduction pathways
\-blood clotting enzymes
\-metabolic pathways that provide energy
\-signal transduction pathways
68
New cards
What are ways to Regulate Enzyme Activity?
\-Irreversible Covalent Activation
\-Irreversible Covalent Inhibition
\-Reversible Covalent Modification of Enzyme
\-Irreversible Covalent Inhibition
\-Reversible Covalent Modification of Enzyme
69
New cards
How does Irreversible Covalent Activation work?
\-usually occurs with proteases
\-referred to as zymogen
\-activated by proteolysis
\-cuts specific peptide bonds
\-referred to as zymogen
\-activated by proteolysis
\-cuts specific peptide bonds
70
New cards
How does Irreversible Covalent Inhibition work?
\-Serpins are used
\-catalytic mechanism will only go through the first few steps
\-catalytic mechanism will only go through the first few steps
71
New cards
How does Reversible Covalent Modification of Enzyme work?
\-amino acids with alcohol side chains can be reversibly phosphorylated
72
New cards
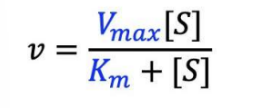
What does everything in this equation mean?
\[S\] = initial substrate concentration
v = initial rate
Vmax = maximum rate
Km = the Michaelis Constant
v = initial rate
Vmax = maximum rate
Km = the Michaelis Constant
73
New cards
What are carbohydrates and functions?
\-hydrated carbon, in formula of (CH2O)n
\-also called sugars or saccarides
\-Functions: chemical energy, biological structures and protein modification
\-also called sugars or saccarides
\-Functions: chemical energy, biological structures and protein modification
74
New cards
What are the functional groups of Monosaccharides?
\-Alcohols(R-OH, saccharides are polyalcohols)
\-Aldehydes(COHR, all bonds off carbonyl cardon are \~120 degrees)
\-Ketones(CORR’, all bonds off carbonyl cardon are \~120 degrees)
\-Aldehydes(COHR, all bonds off carbonyl cardon are \~120 degrees)
\-Ketones(CORR’, all bonds off carbonyl cardon are \~120 degrees)
75
New cards
What happens when alcohols are mixed with either an aldehyde or ketone?
\-with aldehydes, it forms a hemiacetal
\-with ketone, it forms a hemiketal
\-now sp3 hybridized
\-with ketone, it forms a hemiketal
\-now sp3 hybridized
76
New cards
What is the Acid-Catalyzed hemiacetal Formation?
\-aldehyde is protonated and creates oxonium ion
\-an nucleophile is added to the electrophilic carbonyl carbon
\-the oxonium ion is then deprotonated and a hemiacetal is formed
\-an nucleophile is added to the electrophilic carbonyl carbon
\-the oxonium ion is then deprotonated and a hemiacetal is formed
77
New cards
What are sugars?
\-all simple sugars are ketones or aldehydes(ketoses or aldoses)
78
New cards
What is D and L configuration referred to and how is it determined?
\-L is levo(left) and D is dextro(right)
\-refers to the configuration of the highest numbered chiral center only
\-D is most seen in nature
\-refers to the configuration of the highest numbered chiral center only
\-D is most seen in nature
79
New cards
What are the most important pentoses?
\-Ribose
\-Deoxyribose
\-Deoxyribose
80
New cards
What are the most important hexoses?
\-Glucose
\-Mannose
\-Galactose
\-Fructose
\-Mannose
\-Galactose
\-Fructose
81
New cards
What is Hemiacetal and Hemiketal reacting with within a monosacchride?
\-alcohol closest to the chiral center
\-five and six membered rings are most stable
\-this creates an anomeric carbon
\-five and six membered rings are most stable
\-this creates an anomeric carbon
82
New cards
What are the 2 orientation the anomeric carbon can be in?
\-alpha(opposite sides)
\-beta(same sides)
\-beta(same sides)
83
New cards
What are the cyclic structures of monosaccharide structures
\-glucoses predominant form is pyranose
\-fructoses predominant form is furanose
\-fructoses predominant form is furanose
84
New cards
What are ways to represent sugar ring structures?
\-Haworth Projection(good rep and easy to draw, it suggests the ring is flat which it’s not)
\-Chair(more accurate rep and ring is not flat)
\-in a 2D structure and the hydroxyl is either a wedge or dash
\-Chair(more accurate rep and ring is not flat)
\-in a 2D structure and the hydroxyl is either a wedge or dash
85
New cards
What are Phosphate Esters?
\-phosphate group is attaches to carbon and replaces the H on the hydroxyl
86
New cards
What are Sugar acids and Lactones?
\-sugar acids form by the oxidation of the aldehyde, the H on the aldehyde becomes OH(glucuronic acid is by oxidation at carbon-6)
\-lactones form by a hydroxyl group and a carboxylic acid group, making a cyclic ester and releases an OH
\-lactones form by a hydroxyl group and a carboxylic acid group, making a cyclic ester and releases an OH
87
New cards
What are alditols?
\-sugar alcohols are made by a reduction at the aldehyde
\-used for artificial sweetener
\-Glucitol=Sorbitol and is an intermediate transition state for Fructose
\-used for artificial sweetener
\-Glucitol=Sorbitol and is an intermediate transition state for Fructose
88
New cards
What are amino sugars?
\-amino sugars replace an alcohol group with an amino group
89
New cards
What are Glycosides?
\-R3-OH group reacts with hemiacetal or hemiketal and makes it an acetal or ketal
\-the R group will be put first in the name eg. **methyl**-alpha-D-glucopyranoside
\-glycosides from Stable Anomers don’t convert(alpha and beta structures)
\-the R group will be put first in the name eg. **methyl**-alpha-D-glucopyranoside
\-glycosides from Stable Anomers don’t convert(alpha and beta structures)
90
New cards
What are disaccharides and the types of linkages?
\-acetals and ketals formed btwn two monosaccharides
\-linked by a 1,4-glycosidic bond(either alpha or beta linkage)
\-common ones are maltose and cellulose
\-polysacchrides are chains of monosaccharide residue
\-linked by a 1,4-glycosidic bond(either alpha or beta linkage)
\-common ones are maltose and cellulose
\-polysacchrides are chains of monosaccharide residue
91
New cards
What is Cellulose’s and Chitin’s function and the linkage?
\-function is structural support
\-linked by Beta(1,4) bonds
\-chitin has a N-acetylglucosamine on the second carbon
\-linked by Beta(1,4) bonds
\-chitin has a N-acetylglucosamine on the second carbon
92
New cards
What are the functions of Starch, Glycogen, and Glucans and what is the linkage?
\-function is energy storage
\-linked by alpha(1,4) bonds
\-starch is composed of either amylose or amylopectin(has additional alpha(1-6))
\-glycogen resembles amylopectin but has more branches(apha(1.6) branch every 8-12 residues)
\-linked by alpha(1,4) bonds
\-starch is composed of either amylose or amylopectin(has additional alpha(1-6))
\-glycogen resembles amylopectin but has more branches(apha(1.6) branch every 8-12 residues)
93
New cards
What are some properties of the Polysaccharide structures?
\-cellulose is like a sheet, not water soluble and can’t be digested
\-starch and glycogen forms like helix, are water soluble and enzymes can enter, can by digested
\-starch and glycogen forms like helix, are water soluble and enzymes can enter, can by digested
94
New cards
What is an oligosaccharide?
\-short polymer formed by monosaccharide residue linked by glycosidic bonds(2 to several dozen)
95
New cards
What are Glycans and what are some properties?
\-general term that includes both oligosaccharides and polysaccharides
\-can be linear or branched
\-can be composed of a single type or a mixture of different types of monosaccharide
\-can be attached to other biomolecules
\-can be linear or branched
\-can be composed of a single type or a mixture of different types of monosaccharide
\-can be attached to other biomolecules
96
New cards
What are glycoproteins?
\-proteins with attached glycans
\-glycosylation: enzyme-catalyzed addition of glycans to proteins(or lipids)
\-glycosidic bond btwn glycan and certain amino acid
\-two types of glycosylation are O-Linked and N-Linked
\-glycosylation: enzyme-catalyzed addition of glycans to proteins(or lipids)
\-glycosidic bond btwn glycan and certain amino acid
\-two types of glycosylation are O-Linked and N-Linked
97
New cards
What do O-Linked Glycoproteins usually attach to?
\-they attach to N-Acetyl Galactosamine
\-beta-glycosidic bond btwn glycan and a Ser or Thr residue
\-sugar residue that form this bond is N-Acetyl Galactosamine
\-beta-glycosidic bond btwn glycan and a Ser or Thr residue
\-sugar residue that form this bond is N-Acetyl Galactosamine
98
New cards
What do N-Linked Glycoproteins usually attach to?
\-usually uses asparagine
\-usually attach to N-Acetyl Glucosamine
\-the sequence is usually Asn-X-Ser/Thr
\-usually attach to N-Acetyl Glucosamine
\-the sequence is usually Asn-X-Ser/Thr
99
New cards
What is the pattern of N-Linked Glycans?
\-both specific and complex
\-variety of monosaccharide residues and branching patterns
\-the core pattern(image) is further built on by addition of other monosaccharides
\-variety of monosaccharide residues and branching patterns
\-the core pattern(image) is further built on by addition of other monosaccharides
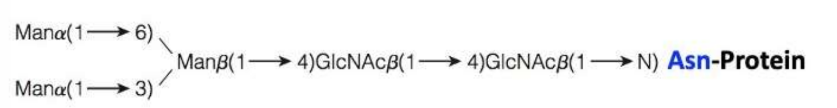
100
New cards
What is the core pattern for IgG Class of N-Linked Glycoproteins?
