Pain (Pathophysiology)
1/94
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
95 Terms
Non Verbal Pain Assessment
Vocal complaints (moaning, groaning, crying out)
Facial grimaces/winces (frowning, grimacing, clenched teeth)
Bracing (holding/protecting a body part)
Restlessness (pacing, fidgeting, shifting in bed)
Rubbing (rubbing or massaging a painful area)
Pain Processing
Transduction
Transmission
Perception
Modulation
Transduction
First step of pain processing
Nociceptor (pain receptor) endings detect harmful stimuli
Injury causes release of pain-producing chemicals
What activates nociceptors?
Mechanical: pressure, swelling, incision, trauma
Thermal: burn, scald, extreme heat/cold
Chemical: toxins, infection, ischemia
What chemicals get released? (Excitatory compounds)
Serotonin
Bradykinin
Histamine
Substance P
Prostaglandins (blocked by NSAIDs)
Where does transduction occur?
In the periphery (at the site of injury)
Transmission
Step 2 of pain processing
Pain signal travels from periphery → spinal cord → brain (thalamus → cortex)
How does the signal travel?
Action potential (pain signal) moves along Aδ fibers and C fibers
Signal enters the spinal cord and ends in the dorsal horn (CNS)
What happens in the dorsal horn?
Excitatory neurotransmitters are released to keep the signal moving upward
Signal travels through ascending pathways toward the brain
Key excitatory neurotransmitters
Glutamate
Neurokinins
Substance P
Where is the signal processed?
Thalamus (but then sent to the cortex to perceive it)
A-δ Fibers
Larger
Myelinated (conducts signals fast)
Faster
Produces “first pain”
Sharp
Stinging
Well-localized
Activated by mechanical or thermal pain
C Fibers
Smaller
Unmyelinated (conducts signals slow)
Slower
Produces “second pain”
Dull
Achy
Burning
Diffuse (hard to localize)
Reaches brain areas involved in emotion
Activated by mechanical, thermal, or chemical stimuli
Where do A-δ Fibers and C Fibers send their signals to?
Dorsal horn of the spinal cord
Where glutamate (for A-δ Fibers) or Substance P (for C Fibers) takes the signals to the brain
Ascending Pain Pathway
The route that the pain signal travels upward from the spinal cord → brain, responsible for carrying pain information so you can feel it
Pain starts at nociceptors (Aδ and C fibers)
Detect painful stimulus
Send signal → dorsal root ganglion
Enter the dorsal horn of the spinal cord
Spinal Cord → Spinothalamic Tract
Pain signal crosses over in the spinal cord
Travels up the spinal cord through the spinothalamic tract
Brainstem (Pons & Medulla)
Midbrain
Thalamus
Receives the pain signal
Sends it to different brain regions:
Somatosensory Cortex (S1) → where you feel and localize pain
Cingulate Cortex → emotional reaction to pain
Limbic System → fear, anxiety, memory of pain
Which chemicals release Nociceptors?
Bradykinin
Cations (protons, potassium ions)
Free radicals (nitric oxide)
Histamine
Prostanoids (prostaglandins, leukotrienes)
Purines (ATP, adenosine)
Serotonin
Tachykinins (Substance P, Neurokinin A)
Super Easy Way to Understand Pain Pathway
Injury → damaged cells release chemicals (bradykinin, histamine, prostaglandins, etc.)
These chemicals activate nociceptors
Nociceptors send signals through:
Aδ fibers (fast)
C fibers (slow)
At the spinal cord, Aδ & C fibers release:
Glutamate (fast pain)
Substance P (slow pain)
Signal goes up spinal cord → thalamus → cortex → you feel pain
Perception
The moment you become consciously aware of pain
Happens when the brain interprets the pain signal
Involves multiple higher brain regions, especially the cortex
Key Brain Areas in Perception:
Reticular System
Somatosensory Cortex (S1)
Limbic System
Reticular System
Controls automatic and motor responses to pain
Example: Pulling your hand away from a hot stove
Part of survival reflexes
Somatosensory Cortex (S1)
Main area where pain is felt and identified
Interprets:
Intensity (how strong)
Type (sharp, dull, burning)
Location (where it is)
Connects pain to past experiences (e.g., “This feels like when I broke my ankle”)
Limbic System
Controls the emotional part of pain
Responsible for:
Fear
Anxiety
Suffering
Memory of pain
Plays a big role in chronic pain and how stressful pain feels
Analgesia
Pain reduction (relief of pain)
Modulation
The descending pain pathway that sends signals down from the brain
Its job is to block or reduce the pain signal in the spinal cord
This process creates analgesia (pain reduction)
The brain releases inhibitory neurotransmitters that STOP or WEAKEN pain signals
These signals travel down to the spinal cord and:
Inhibit Aδ and C fiber transmission
Reduce glutamate and substance P release
“Turn down the volume” on the pain signal
Modulation → Inhibitory Neurotransmitters
Endogenous opioids
Serotonin
Norepinephrine
GABA
Endogenous Opioids
Bind to opioid receptors located in the brain and the spinal cord
Serotonin (5-HT)
Binds to 5-HT and 5-HT3 receptor located in the medulla
Norepinephrine (NE)
Binds to alpha-2 adrenergic receptors, located in the brainstem
GABA
Binds to GABA-A receptors and GABA-B receptors
Modulation → Referred Pain
Pain that starts in one organ or tissue, but is felt in a different location
Why does this happen? (Convergence-Projection Theory)
Somatic pain fibers (from skin, muscles) and visceral pain fibers (from organs) both synapse on the same spinal neuron
Because they share the same ascending pathway, the brain gets “confused” and thinks the pain is coming from the somatic (outer body) area, not the organ
Examples:
Heart attack → pain in left arm
Diaphragm irritation → pain at the tip of the shoulder
Ureteral distension → pain in the testicle
Maladaptive (Pathologic) Pain
Pain caused by damage or abnormal function of the peripheral or central nervous system
Pain persists even when the original injury has healed
Pain becomes a disease itself, not just a symptom
Types:
Neuropathic Pain = nerve damage
Centralized Pain = brain/spinal cord problem
Types of Pain
Nociceptive Pain
Neuropathic Pain
Inflammatory Pain
Nociceptive Pain
Actual tissue injury → activates nociceptors (pain receptors) at the specific site that’s in pain
Types of Nociceptive pain:
Somatic pain
Visceral pain
Somatic Pain
Comes from:
Bones
Joints
Muscles
Skin
Connective tissue
How it feels:
Throbbing
Aching
Sharp or well-localized (you can point to exactly where it hurts)
Visceral Pain
Comes from:
Internal organs
Examples: GI tract, stomach, pancreas, intestines
How it feels:
Can be achy or pressure-like
Can be localized (like a tumor pressing on something)
OR diffuse and crampy (like an obstruction or blockage)
Harder to pinpoint than somatic pain
May cause referred pain (felt in another area)
Neuropathic Pain
Pain caused by damage or disease of the somatosensory nervous system (in the spinal cord)
Neuropathic pain has very distinct sensations:
Burning
Electric / shock-like
Searing
Tingling
Traveling / migrating pain
Types of Neuropathic pain:
Peripheral nerve injury → phantom limb pain
Central nerve injury (spinal cord or brain) → burning, continuous pain
Common causes
Amputation → phantom limb pain
Herpes zoster (shingles)
HIV / AIDS neuropathy
Diabetic neuropathy
Fibromyalgia
Cancer affecting the spinal cord or nerves
Hyperalgesia
Pain feels STRONGER than it should
A stimulus that normally causes mild pain…now causes WAY more pain
Example: A small pinprick feels like a deep stab
Allodynia
Pain from something that should NOT hurt at all
A stimulus that normally does NOT cause pain…now causes pain
Example: A soft blanket feels painful
Peripheral Sensitization
The nerves outside the spinal cord become extra easy to activate, so pain signals fire more often
Here’s what happens:
More ion channels appear: this makes the nerve more reactive
Depolarization threshold is lowered: meaning the nerve fires more easily
Even a small stimulus triggers an action potential → more pain signals sent
Inflammation Pain
When tissue becomes irritated, injured, or infected, and the body launches an inflammatory response
Redness
Swelling
Warmth
Pain
Sometimes loss of function
This inflammation makes nociceptors (pain receptors) more sensitive → so even normal movements can hurt
Example:
Appendicitis
Rheumatoid arthritis
Inflammatory bowel disease (IBD)
Pain Classification
Acute
Chronic
Cancer
Acute → Pain Classification
Short-term pain, caused by something obvious
Lasts less than 3 months
Has a clear cause (surgery, broken bone, infection, injury)
Goes away when the problem is fixed
Chronic → Pain Classification
The pain stays even though the body has healed
Lasts more than 3–6 months
The original injury/illness may be healed, but the pain signals keep firing
Nervous system becomes “sensitized” → keeps sending pain messages
Cancer → Pain Classification
Pain that comes from tumors, cancer treatment, or cancer spreading (metastasis)
Can be acute (new, sudden)
Can be chronic (ongoing)
Neuroplasticity
Injury or inflammation (acute pain)
Pain nerves fire normally
Pain nerves keep firing → become sensitized (subacute pain)
Brain + spinal cord change (“hyperactive”)
Pain continues even after healing (chronic pain)
Pain → Treatment
NSAIDs
Acetaminophen
Aspirin
Alpha-2 Agonists
Anticonvulsants
Antidepressants
Opioid Agonists
Opioid Agonist/Antagonist
Mixed opioid/norepinephrine reuptake inhibitor (NRI) combo
Opioid/non-opioid combo
NMDA antagonist
TRPV1 Agonist
NSAIDs → MOA
Block COX-1 and COX-2 enzymes, which decreases prostaglandins (usually released from nociceptors)
↓ inflammation
↓ pain
↓ fever
BUT ↑ risk of stomach irritation, bleeding, and kidney issues
Acetaminophen → MOA
Works in the brain, not in the peripheral tissues
Inhibits central COX enzymes (weak peripheral activity)
Increases the brain’s pain threshold
Very little anti-inflammatory action
Aspirin → MOA
Irreversibly inhibits COX-1 and COX-2 → ↓ prostaglandins AND ↓ thromboxane (anti-platelet)
Alpha-2 Agonists → MOA
Work mainly in the spinal cord (dorsal horn)
Activate alpha-2 receptors on nociceptive (pain) neurons
↓ norepinephrine release → less pain signal sent to sensory relay neurons
Inhibits pain transmission from nociceptors to the spinal cord relay neurons
Can reduce opioid needs (opioid sparing)
Also lower BP → possible adverse effects: hypotension, bradycardia, sedation
Commonly used in anesthesia for sedation and pain control
Opioid Receptors → MOA
All are G-protein–coupled receptors (GPCRs)
Coupled to Gi (Mu, Delta, Kappa)
Activate K⁺ channels
Inhibit Ca²⁺ channels
Three main types of receptors:
Mu (μ)
Delta (δ)
Kappa (κ)
Pain and Opioid Pathway
Pain starts at the injury site → activates nociceptors (pain-sensing neurons)
Pain signal travels along primary afferent neurons → enters dorsal horn of spinal cord
Signal goes up the spinal cord to the brain (ascending pathway)
Brain can send signals down the spinal cord to reduce pain (descending pathway)
Opioid effects in pathway:
Bind opioid receptors on peripheral nerves, spinal cord, and brain
↓ neurotransmitters (SP, CGRP, glutamate)
↑ potassium channels → hyperpolarization
↓ calcium channels → ↓ pain signal release
Opioid → Side Effects (Dry)
Respiratory depression
Nausea & vomiting
Cough suppression
Sedation / drowsiness
Constipation
Urinary retention
Miosis (pin-point pupils)
Histamine release → itching, hypotension
Morphine = worst
Fentanyl = best (least histamine release)
Respiratory Depression
Seen with all opioids and is dose-dependent
Caused by direct inhibition of brainstem respiratory centers
Mediated by μ-receptor activation in the rostral ventral medulla / rostral dorsal pons
Slows breathing drive → ↓ respiratory rate & depth
Some tolerance develops, but increased doses = higher risk
Reversed with naloxone (Narcan) or naltrexone
The Medulla Oblongata
Nausea & Vomiting
Opioids directly activate the chemoreceptor trigger zone (CTZ) in the medulla
When opioids stimulate CTZ, the brain thinks there is something harmful → leads to nausea + vomiting
This happens with all μ-opioid agonists (morphine, oxycodone, hydromorphone, etc.)
Tolerance often develops → nausea becomes less intense after repeated doses
Mechanism behind CTZ stimulation
μ-receptors in the medulla increase dopamine & serotonin signaling
These neurotransmitters activate the vomiting center
Morphine causes delayed gastric emptying → worsens nausea
Cough Suppression
Opioids depress the cough center in the medulla
This is why codeine is used in cough syrups
μ-agonists decrease the sensitivity of the cough reflex → less coughing
This is separate from respiratory depression but can occur alongside it
Clinical takeaway
Nausea = very common with opioids
Cough suppression = useful therapeutically but can mask symptoms
Both effects originate from medulla oblongata opioid receptor activation
Constipation
Most common chronic opioid side effect
Can be severe enough to require stopping the opioid
Caused by μ-receptor activation in the GI tract
μ-receptors on enteric neurons ↑ K⁺ efflux → ↓ excitability
Leads to less smooth muscle contraction → ↓ peristalsis
Slows GI transit, hardens stool → constipation
Patients often need PAMORs (peripheral μ-antagonists) to manage it
Very little to no tolerance develops → constipation continues even after weeks/months
Miosis
Most μ and κ opioid agonists cause miosis (pin-point pupils)
Happens due to excitation of the parasympathetic nerve that controls pupil constriction
Opioids increase activity of the Edinger–Westphal nucleus → pupil constricts
Minimal tolerance develops; even heavy chronic users often still have pinpoint pupils
Useful diagnostic sign in suspected opioid intoxication/overdose
Mood Alterations
Opioids can cause euphoria, relaxation, tranquility
Mood effects come from dopamine reward pathways, not from the pain pathways
μ-receptor activation → ↑ dopamine release
These pathways mediate reinforcement, contributing to misuse/addiction
Analgesia and euphoria occur through different neural circuits
Opioid Family Groups
Phenanthrenes
Benzomorphans
Phenylpiperidines
Diphenylheptanes
Phenylpropylamines
Phenanthrenes → Drugs
Morphine
Buprenorphine
Butorphanol
Codeine
Dextromethorphan
Dihydrocodeine
Heroin (diacetyl-morphine)
Hydrocodone
Hydromorphon
Levorphanol
Methylnaltrexone
Nalbuphine
Naloxone
Naloxegol
Naltrexone
Oxycodone
Oxymorphone
Phenanthrenes → Drugs (mixed agonist/antagonist → less N/V options)
Buprenorphine
Butorphanol
Dextromethorphan
Hydrocodone
Hydromorphone
Levorphanol
Nalbuphine
Naloxone
Naloxegol
Naltrexone
Oxycodone
Oxymorphone
Benzomorphans → Drugs
Diphenoxylate
Loperamide
Pentazocine
Phenylpiperidines → Drugs
Fentany
Alfentanil
Sufentanil
Remifentanil
Meperidine
Illicit Fentanyl Analogs → Drugs
Furanyl fentanyl
Acetyl fentanyl
Fluoro-fentanyl
Carfentanil
Diphenylheptanes → Drugs
Methadone
Propoxyphene
Phenylpropylamines → Drugs
Tramadol
Tapentadol
Morphine
Has a specific shape and specific chemical pieces that let it bind to the mu-opioid receptor
Small changes to the morphine structure can turn it from an agonist (activator) into an antagonist (blocker)
Opioid receptors bind the (–) version much better
This is why natural morphine (which is the – isomer) works strongly
What it’s used for:
Moderate to severe acute pain
Chronic pain
Pain from myocardial infarction (MI)
Preanesthetic (used before anesthesia to reduce anxiety/pain)
Morpine is glucuronidated in the liver
Forms M6G → ACTIVE metabolite
Low oral bioavailability → need high oral doses
Onset of Action
Oral: ~30 minutes
IV: 5–10 minutes (much faster)
Half-life:
Immediate-release: 2–4 hours
ER formulations: 11–13 hours (last much longer)
Renal Clearance
Must not give to someone w renal issues!!!!
If you tweak morphine slightly → you can create ……………
Antagonists like naloxone or naltrexone
What parts of morphine are needed for mu-agonist activity?
Basic Nitrogen (N)
3-Hydroxy group (phenol)
Aromatic ring (A ring)
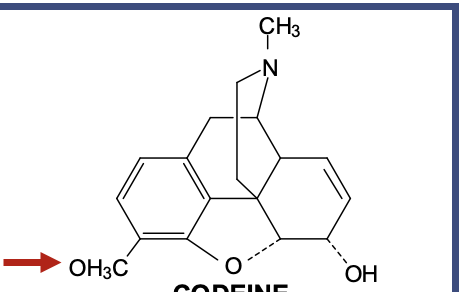
Codeine
Is basically morphine with one small change
That change = at the 3-position, instead of an OH (phenol) like morphine, it has an O-CH₃ (methoxy group)
It makes codeine a weak mu agonist
Morphine needs that 3-OH to strongly bind the mu receptor
Codeine has O-CH₃ instead → weaker binding → weaker analgesia
Antitussive (cough suppressant)
Codeine suppresses cough reflex in the medulla
This effect does not require conversion to morphine, which is why even poor metabolizers still get some cough suppression
Good for mild pain (only) + cough
Good oral absorption
Short half-life (2–3 hrs)
Excreted in urine
Why is Codeine never prescribed for acute pain?
For codeine to work, it needs to be converted to morphine by CYP2D6
Takes too long if person is in pain NOW
Why doesn’t Codeine work in some people?
CYP2D6 (changed codeine to morphine)
People come in different CYP2D6 “types”:
Poor Metabolizers (PM)
Little or no CYP2D6.
They cannot convert codeine → morphine
Ultra-Rapid Metabolizers (UM)
Too much CYP2D6 activity.
Convert codeine → morphine very quickly
How is Codeine converted to Morphine to work?
Must undergo O-demethylation
Full Agonists Opioids
Morphine
Codeine
Hydromorphone
Hydrocodone
Oxycodone
Oxymorphone
Levorphanol
Methadone
Meperidine
Fentanyl
Oliceridine
Opioids → Pregnancy
Opioids cross the placenta
Classified as Pregnancy Category C or D (depends on the opioid)
Opioids → Black Box Warning Pregnancy
Prolonged maternal use of opioids can cause a risk of withdrawal in neonates
Opioids → Interactions
Avoid taking with other CNS depressants
Morphine / Naltrexone Combination
If taken correctly (swallowed whole):
Only the outer ER morphine layer is released
Naltrexone stays trapped and does nothing
If crushed, chewed, injected, or tampered (taken the bad way):
Naltrexone is released
Stops the euphoric effect (pain comes back)
May precipitate withdrawal in opioid-dependent individuals
Onset: ~8 hours
Hydromorphone
Strong opioid for moderate–severe pain
Hydromorphone (Dilaudid) HP = dangerously concentrated → only for opioid-tolerant patients
Metabolized by glucuronidation
Fast onset:
IR oral: 15–30 minutes
IV: ~5 minutes
ER: ~6 hours
Half-life:
IR 2–3 hrs
ER 11 hrs
Multiple routes available
Oral, IV, IM, SubQ, PCA (patient-controlled analgesia), Epidural, Rectal
Is the quickest opioid to work
Can be used in pts w bad renal function, it does not add up inside their body like morphine
Hydrocodone
Moderate to severe pain
Especially when daily, long-term opioid therapy is needed
Alcohol will increase plasma levels or ER formulation
Metabolism: CYP2D6 (changed to hydromorphone) and CYP3A4
Half-life:
8 hours
Hydrocodone IR formulated w/:
Chlorpheniramine → for cough/cold
Pseudoephedrine → decongestant
Ibuprofen → for additional pain/anti-inflammatory effect
Oxycodone
Moderate to severe pain
Often combined with non-opioid analgesics (APAP, ibuprofen, aspirin)
Metabolism: CYP2D6 (changed to oxymorphone) and CYP3A4
Onset of Action
10–15 minutes
Good oral bioavailability
Oxycodone formulated w/:
Acetaminophen
Aspirin
Ibuprofen
Naloxone
Fentanyl
VERY Potent
75–100 times stronger than morphine
Very Lipophilic
Gets into the brain FAST
Used in:
Sedation
Pre-operative use
Anesthesia
Moderate to severe chronic pain
Breakthrough cancer pain (ONLY in opioid-tolerant patients)
Metabolism: CYP3A4
Half Life:
IV: short (2–4 hr)
Patch: long (20–27 hr)
Lozenge/Buccal: moderate (3–14 hr)
Nasal spray: moderate (15–25 hr)
Fentanyl Derivatives
Sufentanil
Alfentanil
Remifentanil
Very potent, Fast onset, Short-acting, Used mainly for anesthesia
Methadone
What it’s used for
Moderate to severe pain
Detox for opioid withdrawal
Maintenance treatment for opioid dependence
Metabolism: Many CYPs, N-demethylation
Half-life:
8–59 hours (HUGE range!)
Increases with repeated doses
Methadone accumulates in the body
Pain relief fades quicker than the drug leaves the body → Risk of unintentional overdose if patients keep redosing
_________________ can prolong the QT prolongation
Methadone
Methadone for Opioid Dependence
Very long acting
Suppresses craving
Smoothes out “peaks and valleys”
Heroin causes:
Big peak → euphoria
Steep valley → withdrawal, cravings
Blocks urge to seek heroin
Because methadone occupies the mu receptor for a long time
Heroin won’t “hit” the receptor as strongly → the high is blocked or reduced
Administered in a highly structured environment
Given at methadone clinics
Patients usually go daily
Allows monitoring for:
Misuse
Diversion
Overdose
QT prolongation
Dosing adjustments
Partial Opioid Agonists
Buprenorpine
Buprenorpine
μ (mu) partial agonist + κ (kappa) antagonist
Used for:
Moderate to severe pain
Opioid dependence
Metabolism: CYP3A4
Onset of Action
IM: ~15 minutes
Half-life:
IV: 2–3 hrs
Sublingual: 37 hrs
Patch: 26 hrs
Excretion
Mostly feces (70%)
Buprenorphine + Naloxone (the yellow pack!)
Used for:
Opioid dependence
Naloxone has very poor oral/sublingual bioavailability
When taken as prescribed (sublingual) → Naloxone does NOTHING
Buprenorphine is the active drug
If someone tries to INJECT it
Naloxone WILL work (because IV = good bioavailability)
It blocks opioids → causes withdrawal
Discourages abuse
Atypical Opioids
Tramadol
Tapentadol
Olicerdine
Tramadol
Used for:
Moderate to severe pain
Weak μ-opioid receptor agonist + SNRI-like action
Tapentadol
What is it used for?
Moderate to severe pain
ER form can treat neuropathic pain from diabetic peripheral neuropathy (DPN)
μ-opioid receptor agonist + Norepinephrine (NE) reuptake inhibitor (NOOO seretonin effects)
Tramadol vs. Tapentadol
Tramadol:
A racemic mixture
→ Contains two enantiomers (mirror-image forms)
Each enantiomer does something different:
Positive (+) enantiomer
→ Must be metabolized (CYP2D6) into the active metabolite M1 to activate the μ-opioid receptor
→ If CYP2D6 is poor → tramadol barely worksNegative (−) enantiomer
→ Responsible for NE reuptake inhibition
→ (Also some serotonin reuptake inhibition → serotonin syndrome risk)
Tapentadol:
Does NOT require metabolism to become active
→ Works immediately in its original form
→ No CYP2D6 dependenceBoth actions come from the SAME molecule:
μ-opioid agonist
NE reuptake inhibitor
Has stronger analgesic potency than tramadol
Olicerdine
A novel IV opioid
A G-protein–biased μ-opioid receptor agonist
Traditional opioids activate TWO pathways:
G-protein pathway → gives pain relief
β-arrestin pathway → causes side effects
Respiratory depression
Constipation
Nausea/vomiting
⭐ Oliceridine mainly activates G-protein, NOT β-arrestin
→ Less β-arrestin recruitment
→ Goal: fewer GI effects and possibly less respiratory depression⚠ BUT: It STILL carries the same black box warnings as all opioids
(respiratory depression, addiction, etc.)Metabolism: CYP 3A4 and 2D6
Opioid Antagonists
Naloxone
Naltrexone
Nalmefene
Naloxone
Reverses opioid overdose, especially respiratory depression
It kicks opioids off the μ-receptor
In people dependent on opioids, naloxone can cause sudden, intense withdrawal (because it rapidly reverses all opioid activity)
Onset: minutes (VERY fast)
Half-life: 20–60 minutes
→ Much shorter than most opioids
→ Must be re-dosed, or patient may “re-sedate” once naloxone wears off
Naltrexone
Used for:
Alcohol dependence
Opioid-induced emergency
Side Effects
Syncope (fainting)
Headache
Nausea / Vomiting
Metabolism
Via dehydrogenase enzymes
Onset of action
Minutes
Half-life
13 hours
Nalmefene
A new opioid antagonist (approved 2023)
Prescription-only
Structurally similar to naltrexone
Reversal of opioid-induced respiratory depression
Works like naloxone, but longer-lasting
Onset of Action
Minutes
Half-life
11 hours
→ MUCH longer than naloxone (20–60 min)
→ Helps prevent re-narcotization after fentanyl or long-acting opioids
Longer duration of action
Higher affinity for opioid receptors
→ Better at reversing potent opioids (fentanyl, analogs)
OPRM1
The gene that codes for the mu-opioid receptor (MOR)
This is the receptor that opioids bind to
Highly polymorphic
More than 200 genetic variants
People naturally differ in how they respond to opioids
What does it bind? 1. Endogenous opioids (your body’s natural pain modulators)
Endorphins
Enkephalins
Dynorphins
2. Exogenous opioids
Morphine
Hydrocodone
Oxycodone
Fentanyl
Heroin
(and all others)
Major role in pain perception
Major role in response to opioid drugs
Some variants may affect how strongly opioids work
(e.g., some people need higher or lower doses)Right now, variations are NOT linked to a specific disease
Not routinely used in clinical decision-making (unlike CYP2D6 or CYP2C19 testing)
COMT
Catechol-O-methyltransferase
An enzyme on nerve terminals
Breaks down neuroamines:
Dopamine
Norepinephrine
Epinephrine
hese neurotransmitters help modulate pain, especially in the descending pain pathway
So COMT activity can influence:
Pain sensitivity
Pain modulation
Response to opioids
Because NE + DA affect pain pathways, differences in COMT may change how people respond to opioids
But this is not clinically actionable yet
Variations exist
No COMT variants are currently linked to any specific disease
Not used in clinical pharmacogenomic testing for opioid prescribing
MTHFR
Enzyme that converts homocysteine → methionine
Methionine is used for:
Protein building
Making neuroamines (dopamine, NE, etc.)
Also helps activate dietary folate
Like COMT, it can influence:
Pain modulation
Pain sensitivity
Opioid response
Because the descending pain pathway is affected by noradrenergic modulation
50–60% of people have reduced MTHFR activity
Reduced activity may influence neurotransmitter levels → may slightly change pain experience
(Not clinically used in opioid prescribing)