Neurophysiology of pain
1/112
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
113 Terms
what is the definition of pain?
“an unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage
• emphasizes how the nervous system and brain interpret and respond to sensory input
integrates biological, psychological, and environmental factors
Mature Organism Model
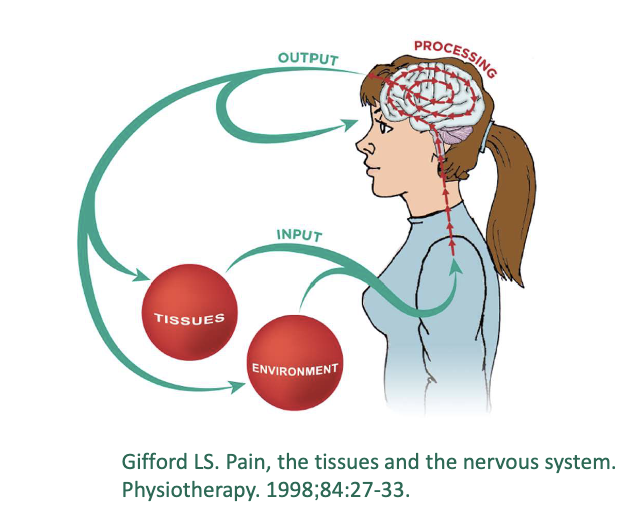
what are key concepts of mature organism model?
input
processing
output
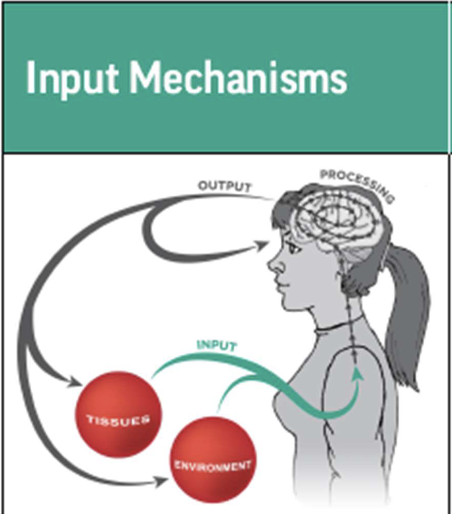
what is input mechanisms ?
Pain begins with nociceptive input from
– peripheral nervous system, outside of the dorsal horn
– peripheral nerves (e.g., C-fibers and Aδ fibers)
– environmental influences
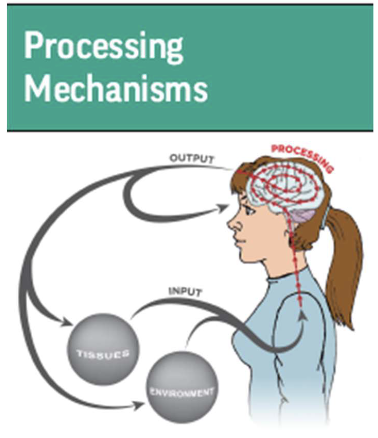
what is processing mechanisms?
Structures and processes inside the CNS
Brain processing the info sent by various inputs
– Sensory
– Cognitive
– Emotional
what is output mechanisms?
response to the input and interpretation of the experience
outputs include:
pain
effects on other biological systems
what happens during injury?
A-delta and C fibers are nociceptive fibers, not “pain fibers”
transmit danger signals (nociception) from injured tissues to the dorsal horn of the spinal cord
These signals are relayed via second-order neurons to the brain,
which decides whether to produce pain
Pain is not directly cause by tissue damage
It is a brain-generated output based on perceived threat
what is low back Input Mechanisms - Tissues and Imaging?
40% of asymptomatic individuals have a bulging disc on MRI
what is neck Input Mechanisms - Tissues and Imaging?
90% of asymptomatic individuals (even in their 20s) have bulging cervical discs
what is shoulder Input Mechanisms - Tissues and Imaging?
After successful rotator cuff surgery and rehab, 90% still show abnormal MRI findings, and 20% retain a complete tear—yet regain function
what is knee Input Mechanisms - Tissues and Imaging?
25–50% of asymptomatic individuals show degenerative changes on MRI.
what is hip Input Mechanisms - Tissues and Imaging?
• 73% of asymptomatic individuals show hip abnormalities on MRI.
• 69% have labral tears without symptoms.
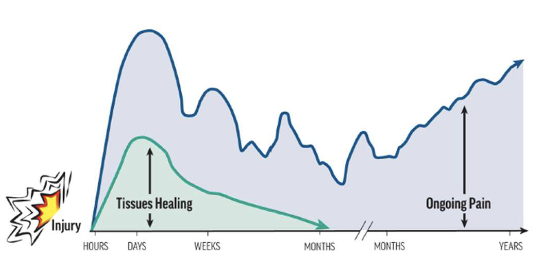
•Tissue injuries, even slow- healing ones like intervertebral discs, can heal over time.
•In the acute/subacute phase, pain may correlate with injury— but in chronic cases, other
actors often dominate.
Clinical Implications – Tissues Injuries
Pain is context-dependent
brain weighs environmental and emotional factors before generating pain
Not all injuries hurt immediately, and not all pain reflects
tissue damage
Therapists should educate patients that:
• Pain is a protective response, not a direct measure of injury.
• Nociception ≠ Pain—they are related but not synonymous.
• Understanding this can reduce fear and improve recovery.
no “pain receptors in the body only __
nociceptors
what do nociceptors do?
– detect and respond to potentially harmful stimuli
– found in skin, muscles, joints, and organs
what do nociceptors respond to?
mechanical
thermal
chemical
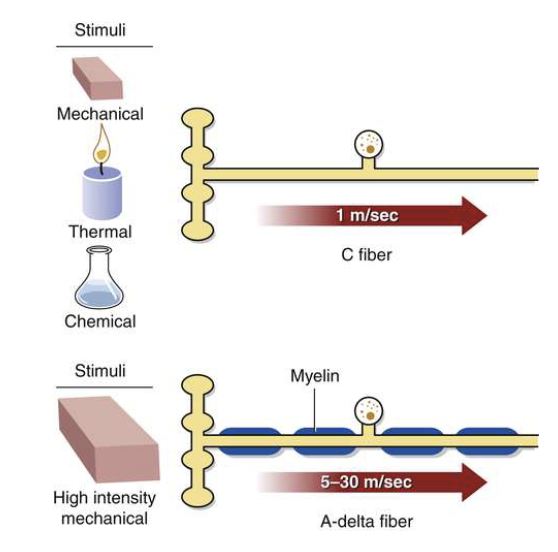
what are types of nociceptors?
C fibers
A delta fibers
what are C fibers?
– Unmyelinated, slow
– Produce dull, aching, poorly defined pain
– Polymodal Receptors
what are a delta fibers?
– thinly myelinated, fast
– Produce sharp, localized pain
– High threshold Mechanical and Mechanical Thermal Receptors
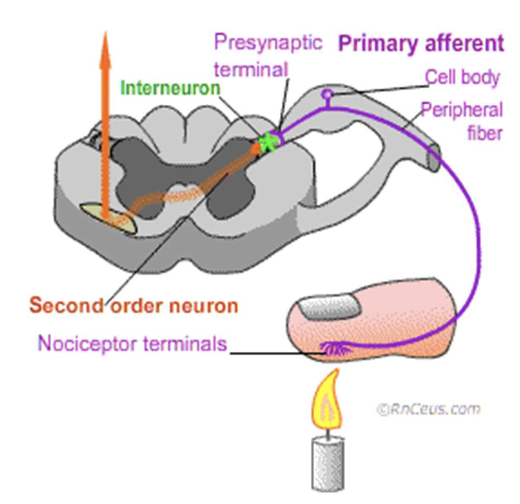
Once activated, nociceptors transmit
electrical impulses through peripheral nerves to the spinal cord, signaling potential harm
Nociception is only the detection of
possible injury and does not necessarily result in pain; the brain ultimately decides if pain is perceived.
stimulus application
activation of peripheral nerve endings
generates action potential
Propagation Along the Axon towards the spinal cord
entry into the SPC
Receptor Pathways - Mechanical and Thermal Stimulation
what is Stimulus Application?
• Mechanical or Thermal
• Activate nociceptors in the skin
what is Activation of Peripheral Nerve Endings
A-delta fibers, C fibers
what is Propagation Along the Axon towards the spinal cord
• A-delta fibers: Fast conduction via thin myelination.
• C fibers: Slow conduction due to lack of myelination
what is entry into the SPC?
Fibers enter via the dorsal root into the dorsal horn of the spinal cord.
chemical stimulus exposure
activation of nociceptors in the skin
generates action potential
signal propagation along the axon towards the SPC
entry into the SPC
Receptors – Chemical Stimulation
what is chemical stimulus exposure?
Endogenous chemicals are released during tissue injury or inflammation
what is Activation of Nociceptors in the Skin
C-fibers
what is generates action potential ?
Chemical binding opens ion channels → influx of Na⁺ and Ca²⁺ → depolarization
what is Signal Propagation Along the Axon towards spinal cord?
C fibers: Slow conduction
what is entry into the SPC?
Fibers enter via the dorsal root into the dorsal horn
– H+ ions, ATP, Serotonin, Substance P- Open Ion Channels
– Bradykinins, Histamines, prostaglandins, Nerve Growth Factors - Increase Nerve Sensitivity
Mast Cells, Macrophages, neutrophiles, T-Cells
what is immune response ?
Release of cytokines and macrophages
chemical release after injury
amplification of pain signals
protective but problematic
Peripheral Sensitization
Chemical Release After Injury
– When tissue is damaged, it releases prostaglandins, bradykinin, histamine, and substance P
– chemicals bind to receptors on nociceptors, lowering their activation threshold
Amplification of Pain Signals:
– nociceptors become hypersensitive
– leads to primary hyperalgesia (increased pain at the site of injury)

Protective but Problematic:
– Initially protective as it encourages rest and healing
– can lead to chronic pain, where nervous system remains in a heightened state of alert
• Injuries don’t occur in isolation—they happen within environments
• Environmental factors can amplify or reduce the pain experience
Input Mechanisms - Environment
environment Influences include:
– Stress, anxiety
– Financial concerns
– Beliefs and fears
– Social and cultural context
– Conditioned response
what is negative environmental influences
what is positive environmental influences
• High-stress environments increase risk of persistent pain
– Car accidents, stressful jobs
Boeing study
what is Boeing study?
– Job satisfaction was the strongest predictor of back pain—not physical workload
what is positive environmental influences?
Early contact sports may reduce risk of chronic pain
Demolition derby drivers: <5% develop chronic whiplash vs. 33% in general population
Cultural stoicism
– Less expressive cultures report less pain
Pain is shaped by biological, psychological, and social factors
Consider the patient’s environment when assessing and treating pain
Clinical Implications – Environment
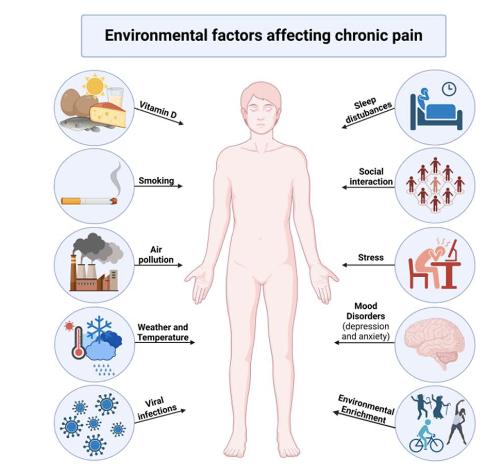
what should be asked about for clinical implications-environment?
• Stress levels
• Job satisfaction
• Cultural background
• Beliefs about pain
Various biological and physiological processes of the __ are important in the development of a pain experience
peripheral nervous system
what are 4 key processes of peripheral neurogenic?
– Ion channel expression
– Nerve compression
– Blood supply
– Dorsal root ganglion (DRG)
None of these processes occur in isolation
Peripheral Neurogenic - Ion Channels
• Ion channels are gateways for ions across nerve membranes
• Crucial for action potential generation and nerve sensitivity
• Targeting ion channels
– Influences the actional potential and sensitivity of the nervous system
• Pharmaceuticals and Therapies can modulate ion channel activity
Ion Channels & Pain – Why They Matter
Proteins forming passages in nerve membranes
Types of ion channels depend on genetic instructions
– Short Half Life (48 hours)
Can open/close to change membrane voltage → depolarization → action potential
Ion Channel Basics
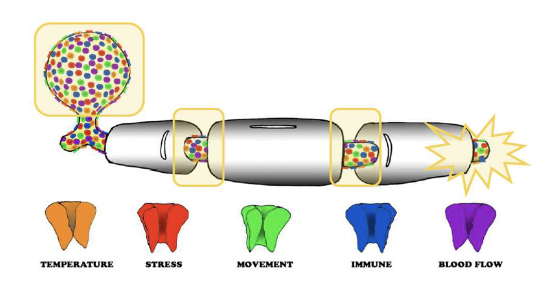
Ion Channel Basics Found in areas of no myelin
– Nodes of Ranvier
– Dorsal Root Ganglia (DRG)
what are types of ion channels?
voltage gated
chemical gated
temperature gated
mechanical gated
immune gated
hydrogen gated
light gated
what is voltage gated?
respond to electrical changes
what is chemical gated
activated by substances like adrenaline
what is temperature gated?
response to heat/cold
what is mechanical gated?
activated by pressure / tension
what is immune gated ?
respond to cytokines
what is hydrogen gated?
sensitive to pH changes
what is light gated?
respond to light exposure
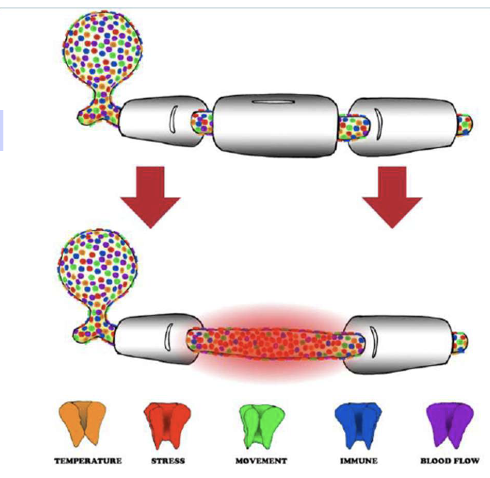
what is myelin loss?
• Mechanical injury (e.g., ankle sprain)
• Immune conditions (e.g., MS, HIV)
• Chemical damage (e.g., inflammation, chemotherapy)
• Create Abnormal Impulse Generating Site (AIGS)
Axons can generate their own impulses when ion channel concentrations are abnormal
Abnormal Impulse Generating Sites (AIGS)
Abnormal Impulse Generating Sites (AIGS) Can be triggered by:
• Stress (adrenaline, fear, anxiety)
• Movement or pressure
• Temperature changes
Therapy can modulate ion channel activity
Pain Neuroscience Education (PNE)
Therapeutic alliance
Mindfulness
These reduce stress chemicals (catecholamines) → lower ion channel expression → reduced sensitivity
Clinical Implications - Therapy
Mechanical compression of nerves typically causes:
Radiculopathy Insight:
Pathophysiological Cascade:
Peripheral Neurogenic - Nerve Compression
Mechanical compression of nerves typically causes:
• Numbness
• Weakness
• Pins & needles
• Not pain directly
Radiculopathy Insight:
Pain in extremities often due to chemical activation of
Pathophysiological Cascade:
1. Compression → altered blood flow/nutrition → pain
2. Neurogenic inflammation → macrophages & T-lymphocytes
3. Demyelination → ion channel influx -> AIGS
Peripheral sensitization
Nervous system = 2–3% of body mass
Consumes ~25% of circulating oxygen
6–8% stretch → slowed blood flow
15% stretch → blood flow stops
20% stretch → cell death & demyelination
Peripheral Neurogenic - Blood Flow
blood flow clinical implications:
• Reduced blood flow → peripheral sensitization
• Increased blood flow → desensitization
• Cluster of sensory neuron cell bodies
• Located outside the spinal cord
• Non-myelinated → high concentration of ion channels
Peripheral Neurogenic - Dorsal Root Ganglion (DRG)
what are key features:
• Highly mechanosensitive
• Sensitive to stress chemicals
• Referred to as "the most sensitive structure in the human body"
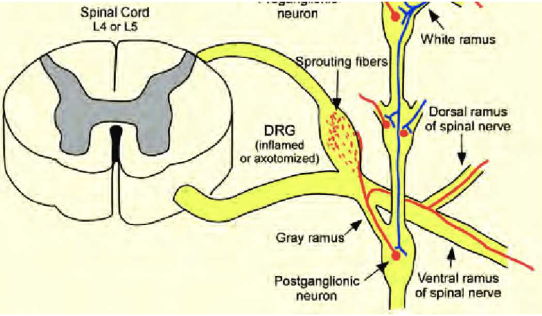
Sympathetic fibers form a basket weave around the DRG
Releases adrenaline → triggers action potentials fires bi- directionally
Antidromically → target tissues → release of substance P, histamine → redness, swelling, spreading pain → peripheral sensitization
Orthodromically → CNS → central sensitization
Clinical Implications - DRG
Pain processing is complex at each spinal level due to:
• Convergence from adjacent spinal segments
• Sympathetic nervous system input
• Immune system activity
• Motor neuron feedback
• Contralateral (opposite side) input
Gating mechanisms ensure accurate transmission of
Location (e.g., dermatome)
Type of stimulus (e.g., light touch vs. danger)
Side of the body
what is complex process?
“feature exact”
indicated health nervous system
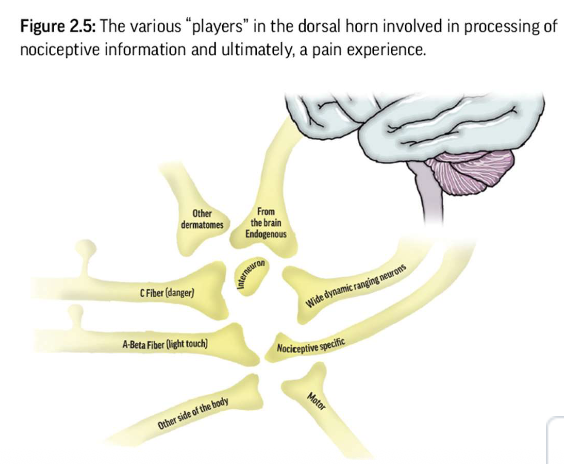
Receives nociceptive input from peripheral tissues via
nociceptive fibersIncoming signals are processed by interneurons
inhibit
facilitate to second order neurons
Processing Mechanisms - Dorsal Horn
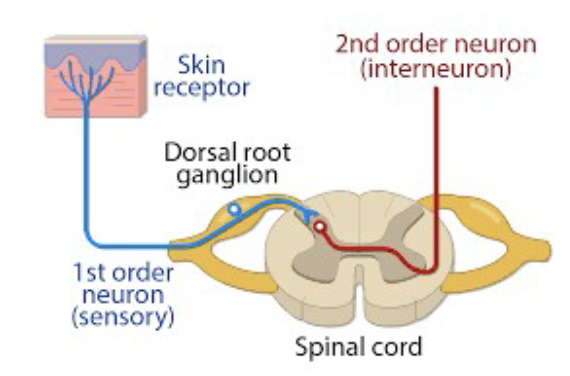
transmit nociceptive information to the brain for interpretation and response
Processing Mechanisms - Second Order Neurons
Two key types of second-order neurons:
Wide Dynamic Range (WDR) neurons: respond to a range of stimuli; involved in everyday sensory processing
Nociceptive Specific (NS) neurons: respond only to high-threshold, noxious stimuli; activated in severe threat scenarios
A-beta fibers transmit non-noxious sensory information (e.g., light touch).
Processing Mechanisms – Spinal Cord
A-beta fibers transmit non-noxious sensory information (e.g., light touch).
Processing Mechanisms – Spinal Cord
pants brushing the medial knee sends signals via L3 dorsal horn
Processing Mechanisms – Spinal Cord example
These signals are often blocked at the spinal cord level by interneurons, preventing cortical awareness
– Neurotransmitters involved
• GABA and glycine – inhibitory
• Glutamate– excitatory

Periaqueductal Gray (PAG)
Relay through Brainstem Nuclei
Neurotransmitter Release
Endogenous opiod release
opioid receptor activation
pain signal inhibition
Pain Modulation - Descending Pathway
what is PAG?
– Located in the midbrain
– Receives input from the cortex and limbic system (e.g., amygdala, hypothalamus)
what is relay through brainstem Nuclei?
Locus Coeruleus (LC) – releases norepinephrine (noradrenaline)
what is Neurotransmitter Release?
– Serotonin and norepinephrine descend to the spinal cord
– Modulate pain by acting on interneurons in the dorsal horn
what is endogenous opiod release?
Includes endorphins, enkephalins, dynorphins
what is Opioid Receptor Activation?
– Located presynaptically (on nociceptor terminals) and postsynaptically (on second-order neurons)
– Inhibits release of substance P and glutamate
– Reduces excitability of pain-transmitting neurons
what is Pain Signal Inhibition?
– Centrally: Inhibits transmission in the spinal cord
– Peripherally: Reduces nociceptor sensitivity
Repeated stimulation of dorsal root afferents (especially C fibers) causes action potential windup
Windup = progressive increase in neuron firing due to temporal summation
Persistent input leads to neuroplastic changes in spinal cord and brain → central sensitization
– Chronic C fiber input can cause interneuron death
– Loss of interneurons reduces inhibitory control, increasing nociceptive signal transmission to the second order neuron
Clinical Implications – Central Sensitization
Persistent nociceptive input causes reorganization of dorsal horn laminae
C fibers retract, and A-beta fibers grow into nociceptive layers, allowing light touch to activate pain pathways → light touch allodynia
Neuroplastic Changes in the Dorsal Horn
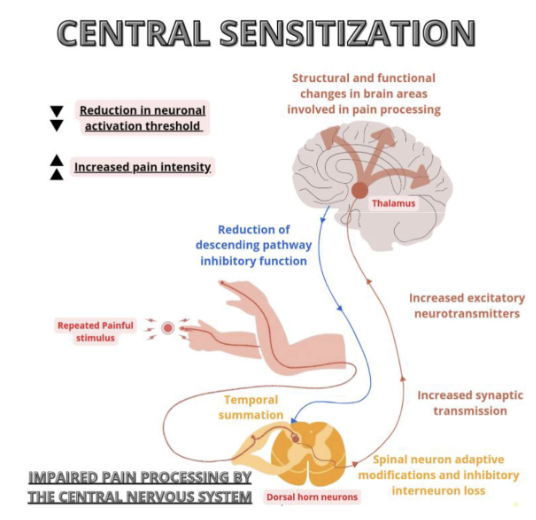
Central Sensitization
With fewer interneurons
Brain receives more signals, but with less precision
Results in increased threat perception and reduced descending inhibition
Clinical Implications - Feature Loss
With fewer interneurons
– removes gating mechanism
– allows input from multiple sources (other spinal levels, opposite side of body, A-beta fibers) to reach second-order neurons
– brain receives mixed signals (e.g., pain and touch)