Characteristics of Groups in the Periodic Table
1/69
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
70 Terms
Group on the Periodic Table
Elements in a group have similar chemical properties due to the same number of valence electrons.
Atomic Number
Number of protons in an atom, which determines the nuclear charge and the arrangement of the Periodic Table.
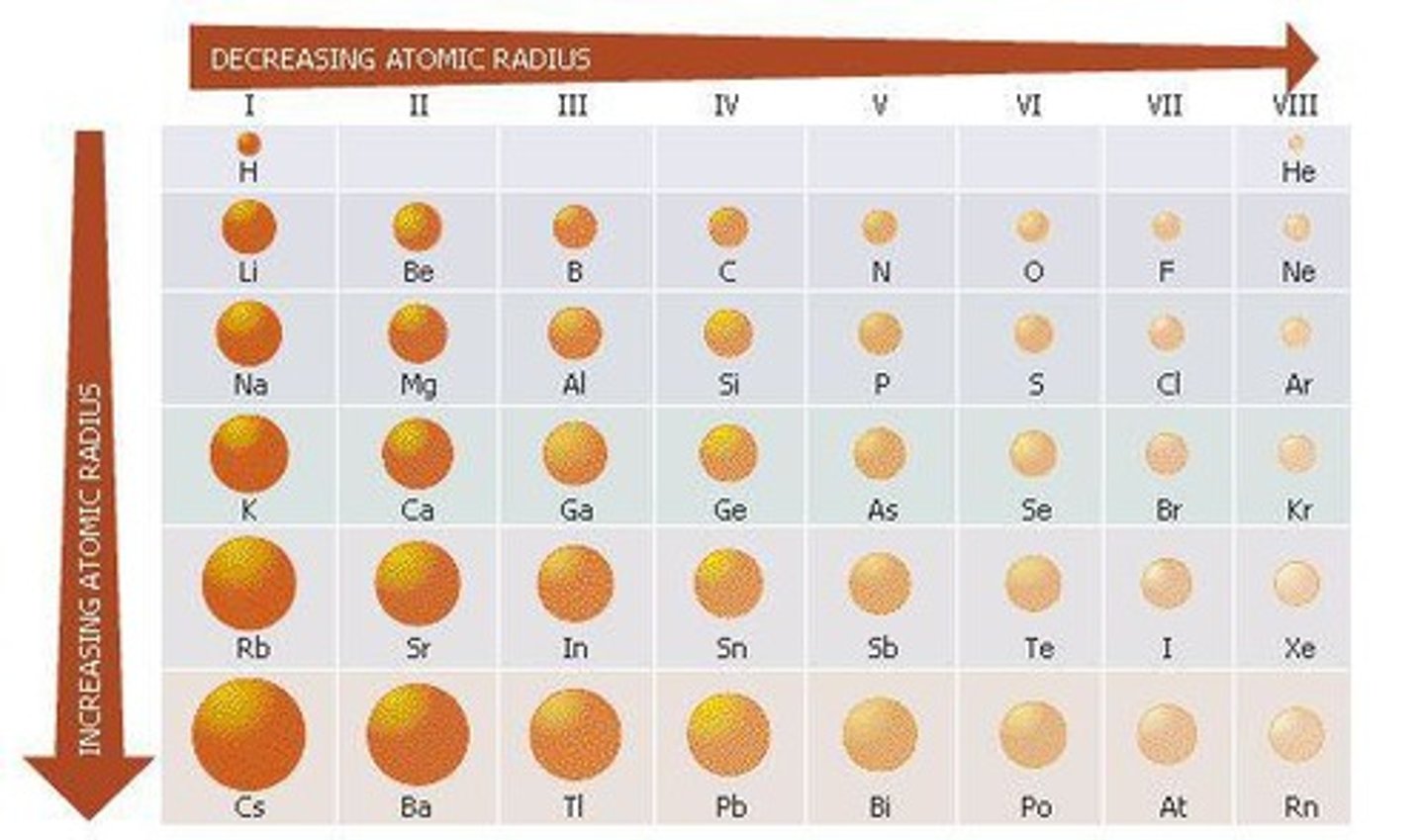
Atomic Radius
Distance between the nucleus and the farthest electron.
Atomic Radius Group Trend
Atomic Radius increases as you go down the group due to more occupied principal energy levels.
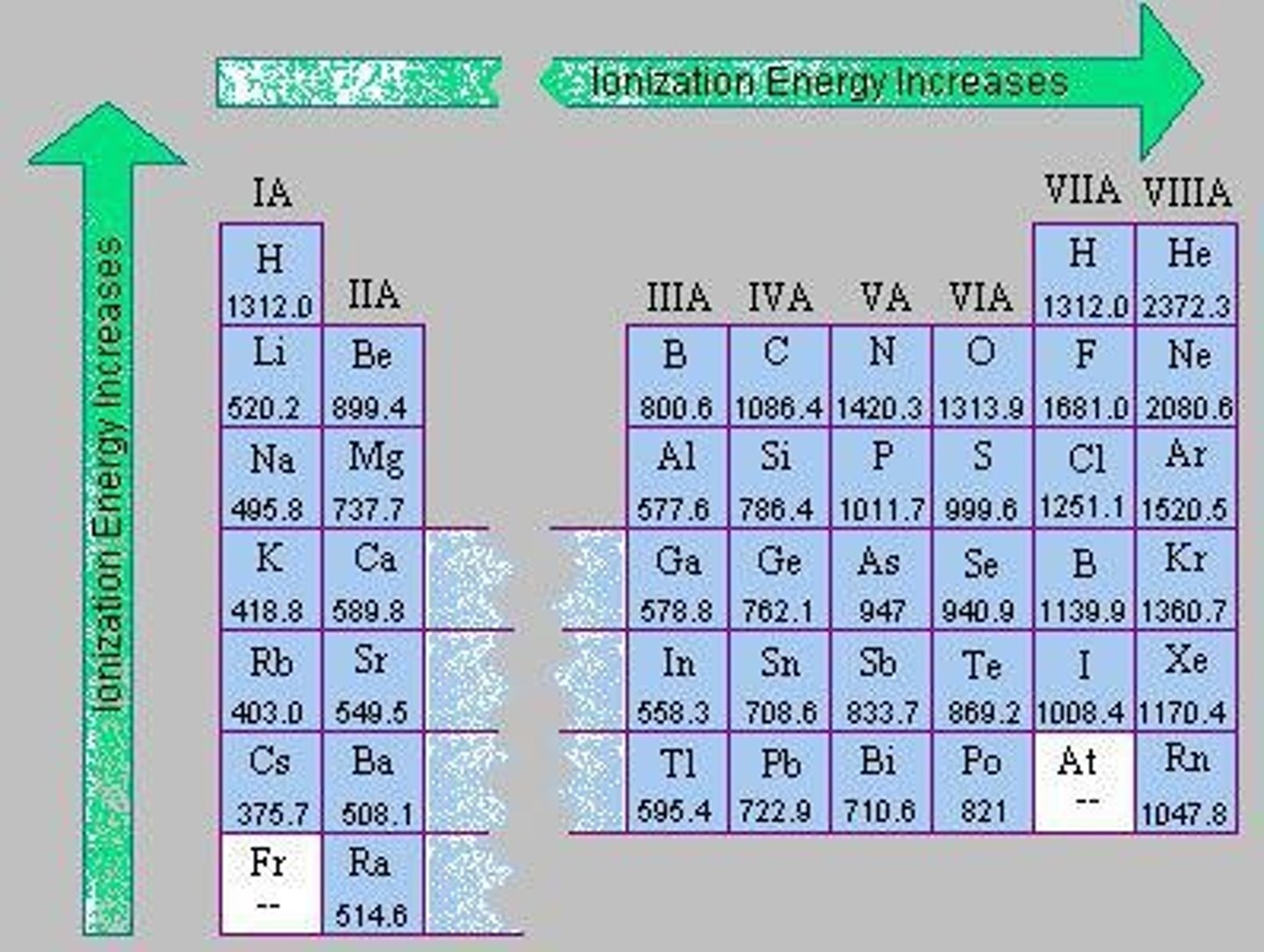
First Ionization Energy (IE)
The amount of energy needed to remove the most loosely bound electron from the valence shell.
Ionization Energy Trend
Ionization Energy decreases as you go down a group because larger radius atoms have valence electrons farther from the nucleus.
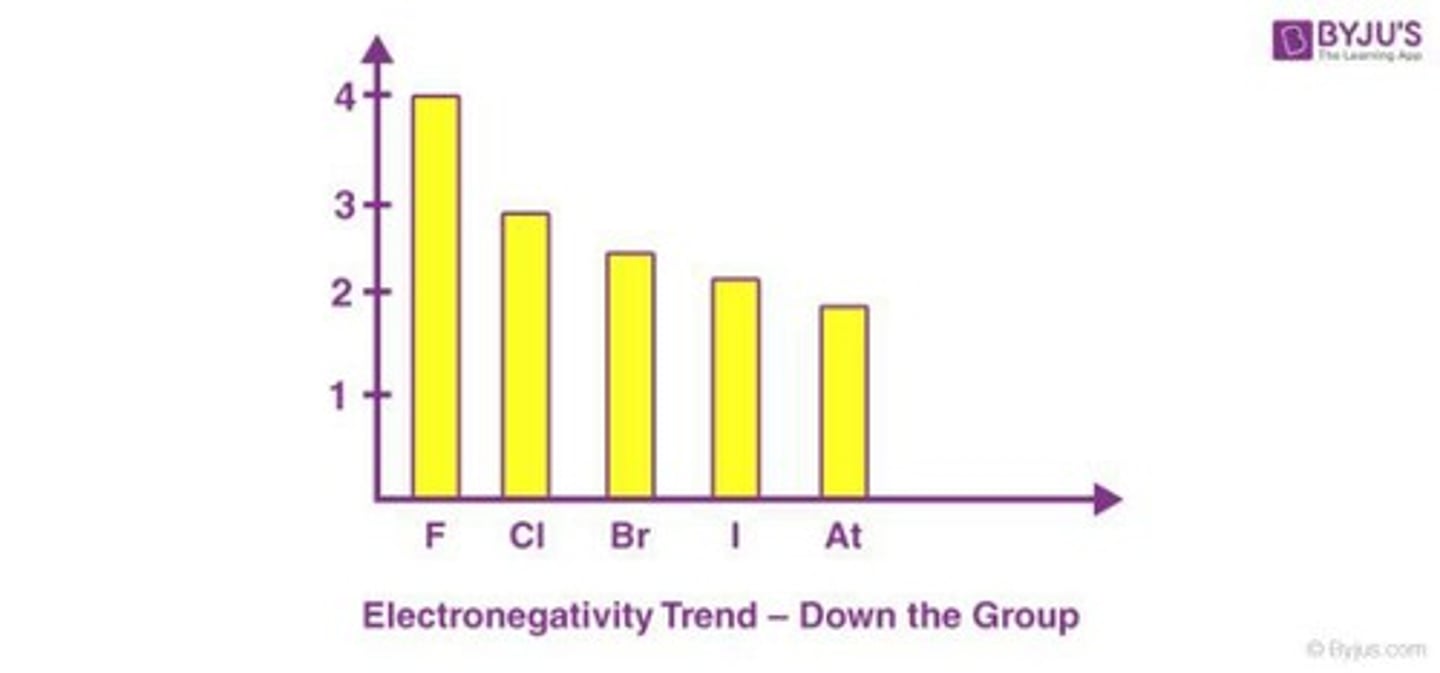
Electronegativity (EN)
A measure of the attraction of a nucleus of one atom for another atom's valence electrons.
Electronegativity Group Trend
Electronegativity decreases as you go down the group due to larger atomic radius.
Metallic Property
Measure of how easily an element loses electrons.
Metallic Property Group Trend
Metallic properties increase as you go down the group.
Example of Group 1 Compound
Na+1 Cl-1 → NaCl
Example of Group 13 Compound
B+3 O-2 → B2O3
Atomic Radius Example
Li = 130 pm, Cs = 238 pm
Group IA Ionization Energy Example
Li = 520 KJ/mol, Fr = 393 KJ/mol
Electronegativity Scale
A man-made scale from 0 to 4.
Group Review Question 1
Which of the following has the highest electronegativity; Ca or Ba? Why?
Group Review Question 2
Which of the following is most metallic; Li or Rb? Why?
Group Review Question 3
Which pair of elements have most similar characteristics; K & Cl, K & Ca, or F & Cl? Why?
Group Review Question 4
Which of the following has the lowest Ionization Energy; Ca or Ba? Why?
Diatomic Element
An element that exists as a molecule composed of two atoms.
Element with greatest metallic character in Group 15
Bismuth (Bi)
XO formation
Metal 'X' comes from Group 2.
Element in group 2 with largest electronegativity
Magnesium (Mg)
Element in group 3 with smallest ionization energy
Aluminum (Al)
Group 1
Alkali Metals - Extremely Active, Form Strong Bases.
Group 2
Alkaline Earth Metals - Very Active; but not as much as the Alkali Metals.
Properties of Groups 1 & 2
Found only in compounds in nature, are the most active metals on the Periodic Table, lose electrons easily.
Low Ionization Energy
A characteristic of elements in Groups 1 and 2.
Low Electronegativity
A characteristic of elements in Groups 1 and 2.
Ionic Compounds
Compounds formed by elements in Groups 1 and 2.
Transition Metals
Elements in Groups 3 to 12 that have multiple oxidation numbers.
Colored Ions in Water
Transition metals can form compounds which have colored ions in water.
Elemental Transition Metals
Examples include Gold (Au), Copper (Cu), and Silver (Ag).
Mercury (Hg)
The only metal that is liquid at room temperature.
Halogens
Group 17 elements that all have 7 valence electrons and gain 1 electron to become -1 ions.
Phosphorus
Not a diatomic element.
Nitrogen
A diatomic element.
High Electronegativity
Gain another atom's electrons easily
High Ionization Energy
Do not lose their electrons easily
Noble Gases
All are Non Metals & Gases at Room Temp
Noble Gases Reactivity
DO NOT react with other elements because they have filled/stable outer shell
Valence Electrons in Noble Gases
8 valence electrons = full shell, except He which has 2 valence electrons = full shell
Characteristics of a Period
Elements in the same period have the same number of occupied Principal Energy Levels (PEL's).
Period 3 Elements
All have 3 occupied rings.
Atomic Number Trend in a Period
Atomic Number Increases as one moves across a period.
Valence Electrons Trend in a Period
Number of Valence Electrons Increase as one moves across a period.
Atomic Radius Trend in a Period
Atomic Radius Decreases as one moves across a period due to greater nuclear charge.
Atomic Radius Values
Na: 190, Mg: 160, Al: 143 (in pm) showing more protons pull electrons closer to the nucleus.
Ionization Energy Trend in a Period
Ionization Energy Increases as one moves across a period, requiring more energy to remove electrons from non-metals than metals.
Electronegativity
The attraction for another atom's electrons increases.
Atomic radius
Decreases; atom's nucleus is closer to another atom's valence electrons.
Nuclear Charge
Greater nuclear charge causes electrons to be held closer to the nucleus due to a smaller radius.
Metals
Elements that lose electrons to become (+) ions; have properties like luster, ductility, malleability, and good conductivity.
Non-Metals
Elements that gain electrons to become (-) ions; most are gases, some are solids, and they lack luster and are poor conductors.
Metalloids
Elements that have some properties of both metals and non-metals; located at the staircase between metals and non-metals.
Ionic Radius
For metals, it is smaller than atomic radius; for non-metals, it is larger than atomic radius.
Most Active Metals
Found lower and to the left of the periodic table; Francium (Fr) is the most reactive metal.
Most Reactive Non-Metal
Fluorine (F) is the most reactive non-metal.
Properties of Metals
Have luster, are ductile, malleable, good conductors of electricity and heat, and are all solids at room temperature except mercury (Hg).
Properties of Non-Metals
High electronegativity (E.N.), high ionization energy (I.E.), most are gases, and Br is the only liquid non-metal.
Properties of Metalloids
Elements that are malleable and have poor conductivity; examples include B, Si, As, Te, Ge, Sb.
Ductile
Can be drawn into a wire.
Malleable
Can be hammered into shape.
Good Conductor
Ability to conduct electricity and heat effectively.
Luster
Shine or reflective quality of a surface.
Brittle
Tendency to break or shatter easily.
Liquid Non-Metal
Bromine (Br) is the only liquid non-metal.
Solid Non-Metal
Iodine (I) is a solid non-metal at room temperature.
Low Ionization Energy
Characteristic of metals, indicating they lose electrons easily.
Low Electronegativity
Characteristic of metals, indicating a weak attraction for electrons.