To determine the amount of water crystallisation in washing soda
0.0(0)
Card Sorting
1/9
There's no tags or description
Looks like no tags are added yet.
Last updated 10:41 AM on 5/6/25
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
10 Terms
1
New cards
Draw a diagram of this experiment
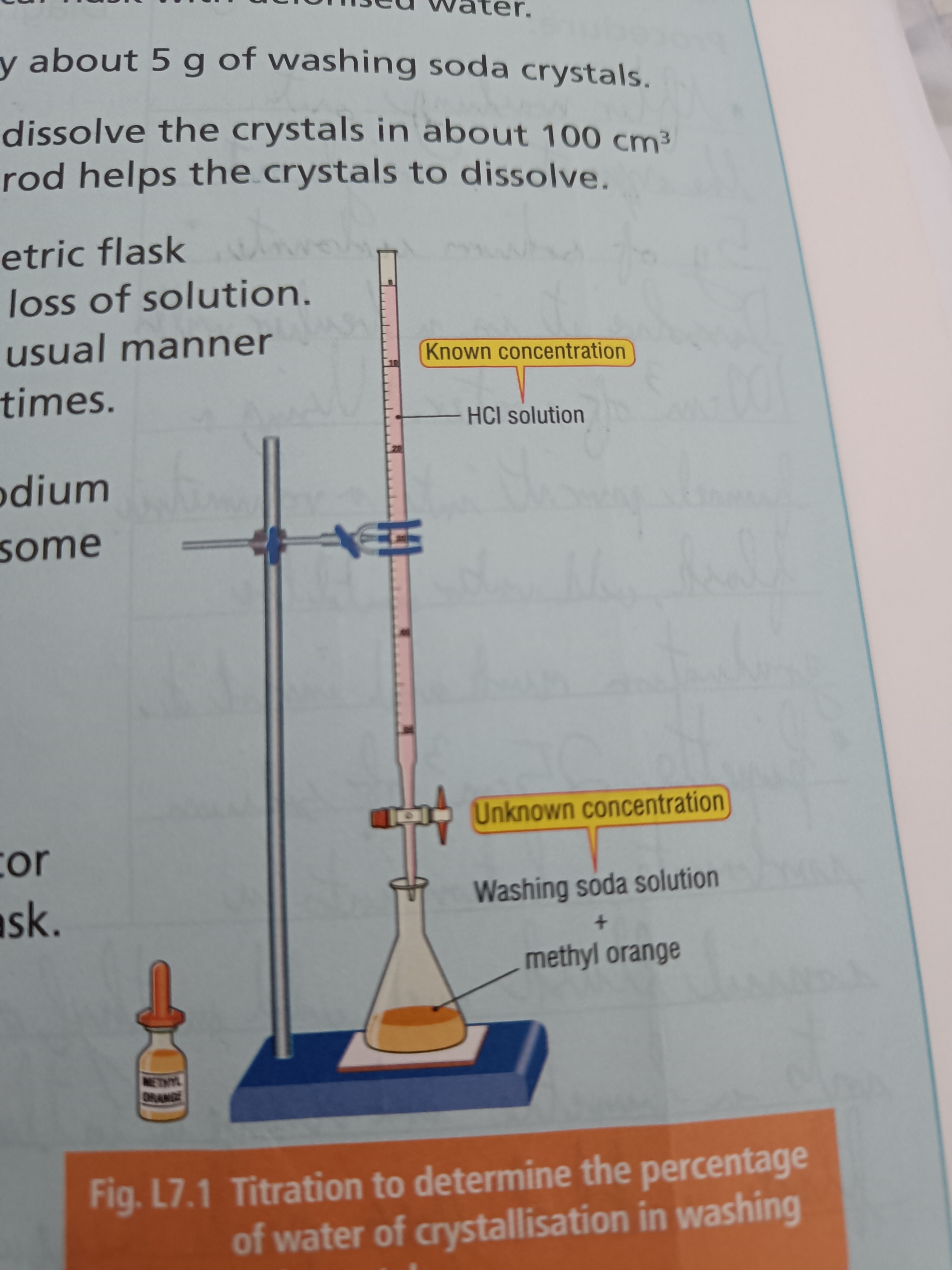
2
New cards
Step 1
Weigh sodium carbonate and dissolve it in a beaker of water.
3
New cards
Step 2
Pour it into a volumetric flask using a funnel
4
New cards
Step 3
Add water until it reaches the graduation mark and invert it
5
New cards
Step 4
Pipette sodium carbonate solution into a conical flask.and add methyl orange
6
New cards
Step 5
Pour HCl into a burette with a funnel and drip it into the conical flask until it turns pink
7
New cards
Step 6
Repeat it 2 more times
8
New cards
Why is the volumetric flask inverted?
Ensures solution is homogenous
9
New cards
Colour change of this experiment
Orange to pink
10
New cards
Why is deionised water used instead?
Tap water may have dissolved ions that may affect pH of the solutions