Molecular Geometry: Bond Angles (with pictures)
1/28
Earn XP
Description and Tags
exam 4, chem 115, WVU fall '25
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
29 Terms
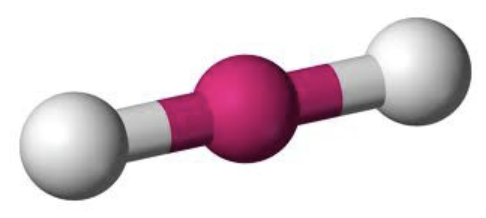
AX₂ shape
linear
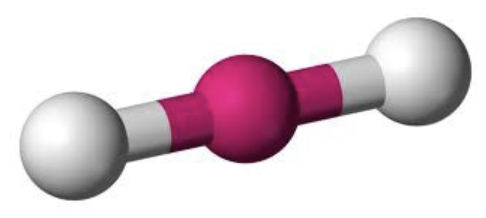
AX₂ bond angle
180
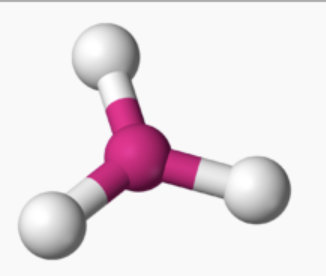
AX₃ shape
trigonal planar
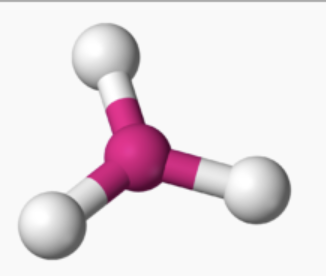
AX₃ bond angle
120

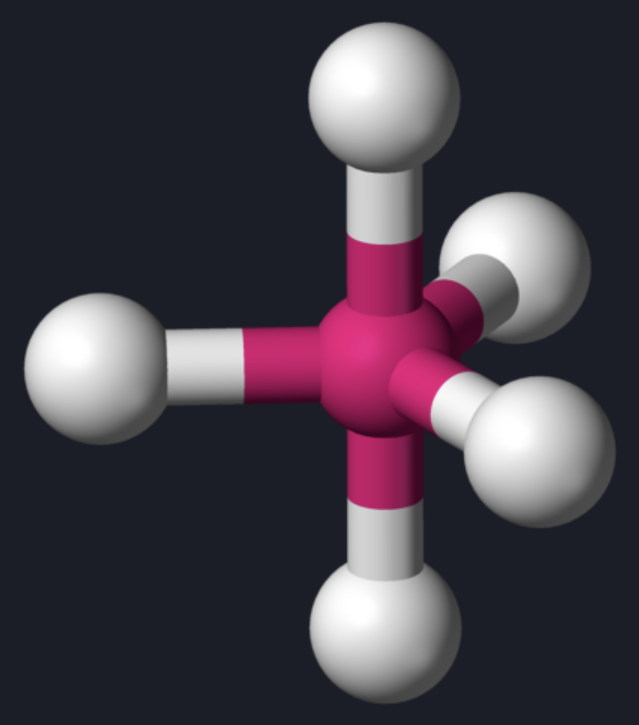
AX₄ shape
tetrahedral
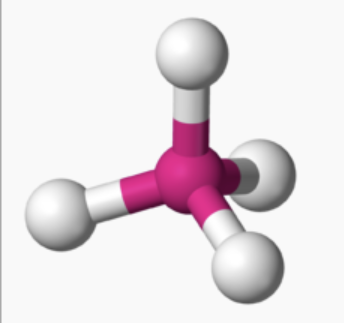
AX₄ bond angle
109.5
AX₅ shape
trigonal bipyramidal
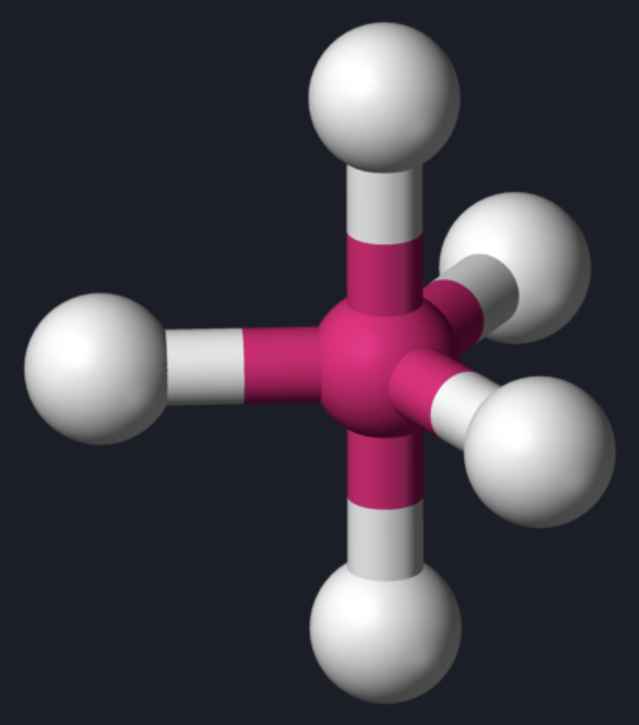
AX₅ bond angle
90 (axial), 120 (equatorial)
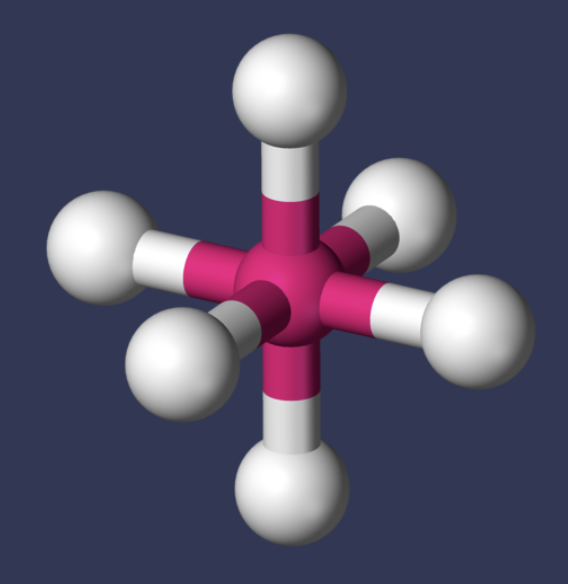
AX₆ shape
octahedral
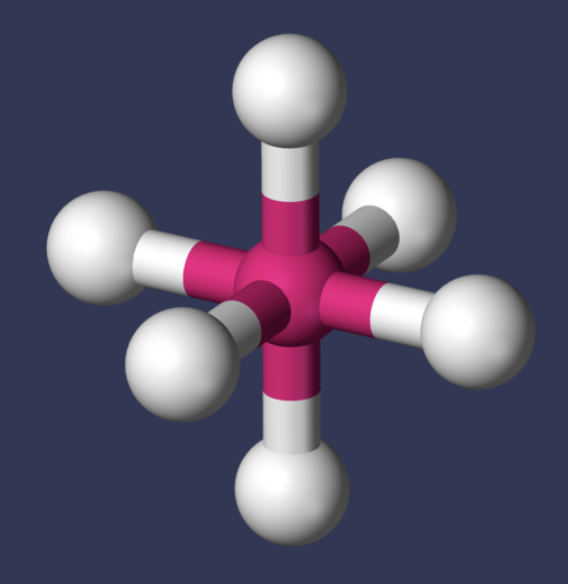
AX₆ bond angle
90
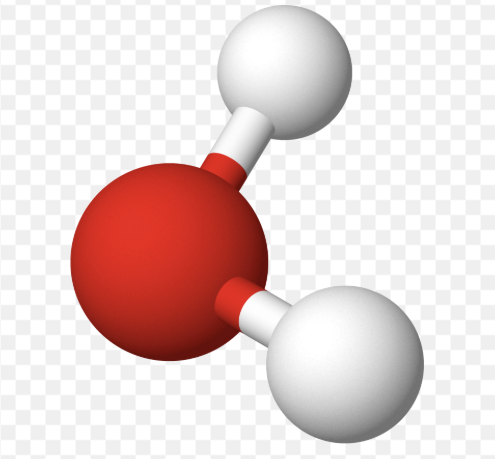
AX₂E₁ shape
bent
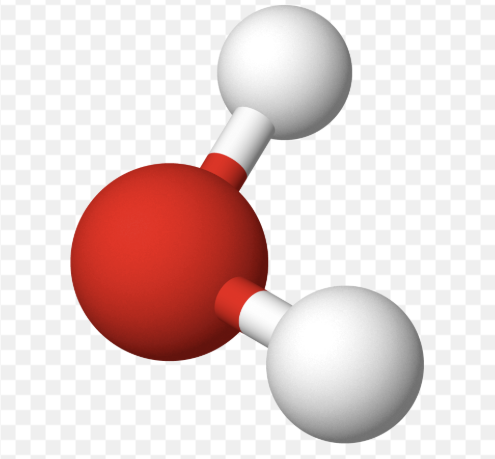
AX₂E₁ bond angle
119
AX₂E₂ shape
tetrahedral, nonlinear bent
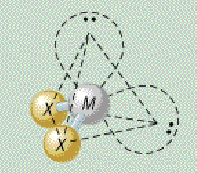
AX₂E₂ bond angle
109.5
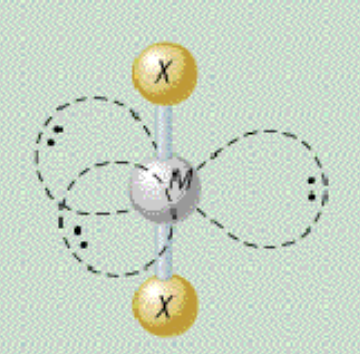
AX₂E₃ shape
trigonal bipyramidal, linear
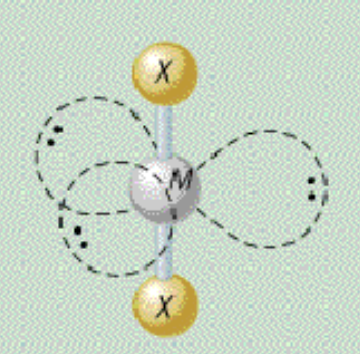
AX₂E₃ bond angle
180
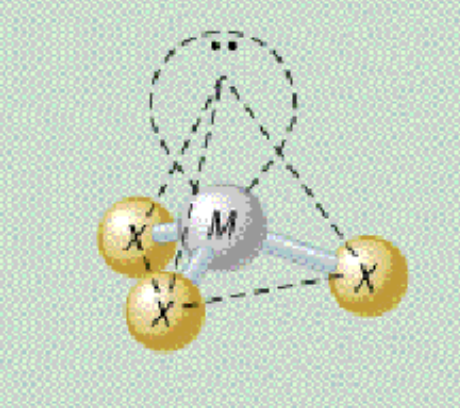
AX₃E₁ shape
trigonal pyramidal
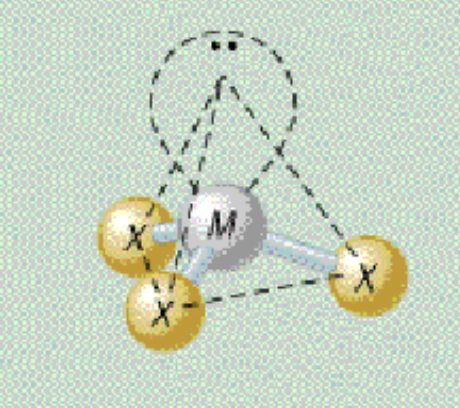
AX₃E₁ bond angle
109.5
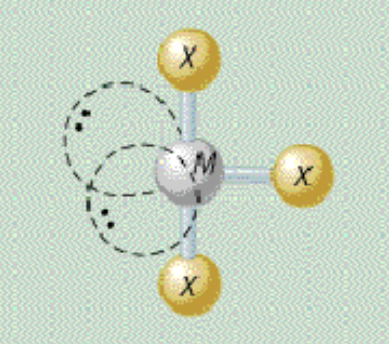
AX₃E₂ shape
trigonal bipyramidal, T-shaped

AX₃E₂ bond angle
less than 90, less than 180
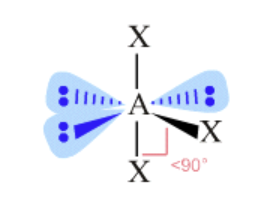
AX₃E₃ shape
octahedral, T-shaped
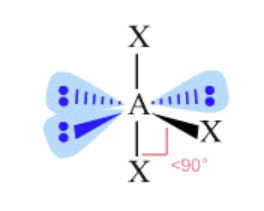
AX₃E₃ bond angle
less than 90, greater than 180
In trigonal bipyramidal molecules, ___ positions are taken first by valence electrons
equatorial

AX₄E₁ shape
distorted tetrahedral
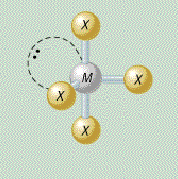
AX₄E₁ bond angle
less than 90, less than 120
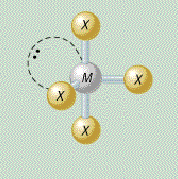
AX₄E₂ shape
square planar
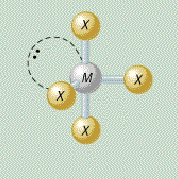
AX₄E₂ bond angle
less than 90
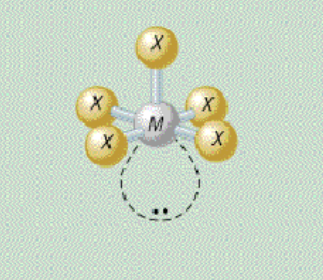
AX₅E₁ shape
square pyramidal
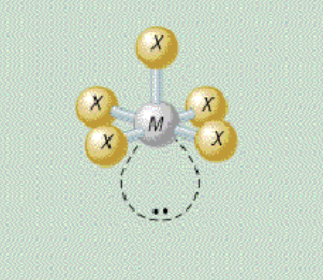
AX₅E₁ bond angle
90