Organic Chemistry IUPAC Naming Conventions
1/16
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
17 Terms
List the steps of IUPAC nomenclature
1. Identify the longest carbon chain containing the highest-order functional group
2. Number the chain
3. Name the substituents
4. Assign a number to each substituent
5. Complete the name
- alphabetical order (di, tri, etc. not included)
*(iso, neo, etc. are included)
-named based on backbone change and based on highest priortity
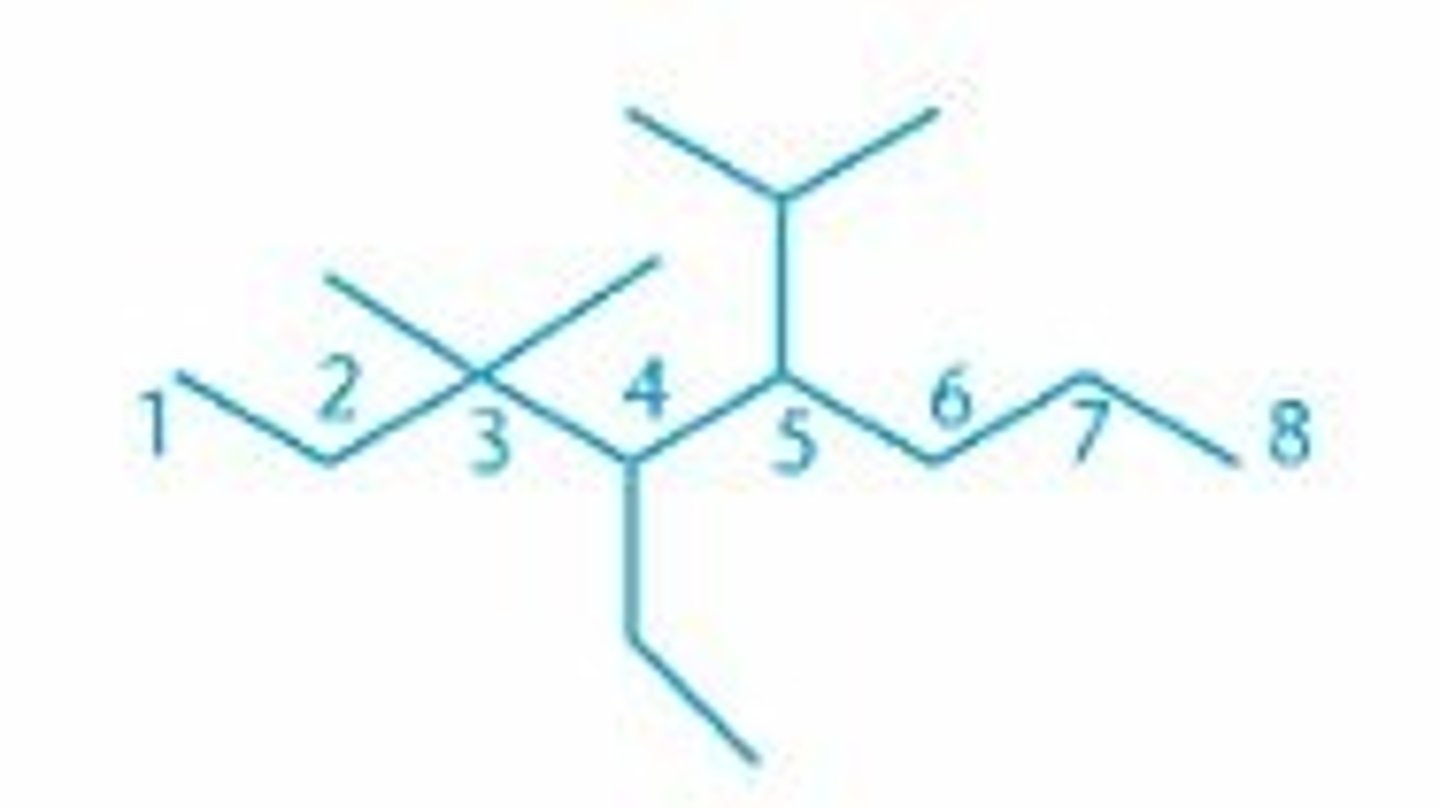
Name this molecule
4-ethyl-5-isopropyl-3,3-dimethyloctane
What are hydrocarbons?
compounds that contain only carbon and hydrogen
What is the formula for alkanes
CnH(2n+2)
List the name of alkanes from total carbons 1 -8
methane
ethane
propane
butane
pentane
hexane
heptane
octane
In naming alcohols, if you have a double or triple bond, which of these three things takes priority in naming?
The alcohol
If a carbon chain has an alcohol that is not the highest-priority functional group, what is it named as?
A hydroxy substituent
(hydroxy-)
What are alcohols with two hydroxyl groups called? What is their suffix?
Diols or glycols
(-diol)
What is the difference between geminal diols and vicinal diols?
geminal diols: hydroxy groups on the same carbon
vicinal diols: hydroxy groups on adjacent carbons
What are the common names for 2-propanol and ethanol?
isopropyl alcohol
ethyl alcohol
What is the difference between an aldehyde and a ketone?
aldehyde is a carbonyl group at the end of a chain
ketone is a carbonyl group in the middle of a chain
What suffixes are used for aldehydes and ketones; how are carbonyl groups named as a substituent?
aldehyde suffix: aldehyde prefix:
ketone suffix: ketone prefix:
aldehyde: -al, oxo-
ketone: -one, keto- or oxo-
What are the common IUPAC names for the following compounds:
methanal
ethanal
propanal
propanone
formaldehyde
acetaldehyde
propionaldehyde
acetone
For a molecule with a double bond, an aldehyde, and an alcohol, which functional group would determine the suffix when naming?
aldehyde
In a molecule with two double bonds adjacent to each other and an alcohol, which functional group would take precedence in naming?
alcohol because of its higher oxidation state
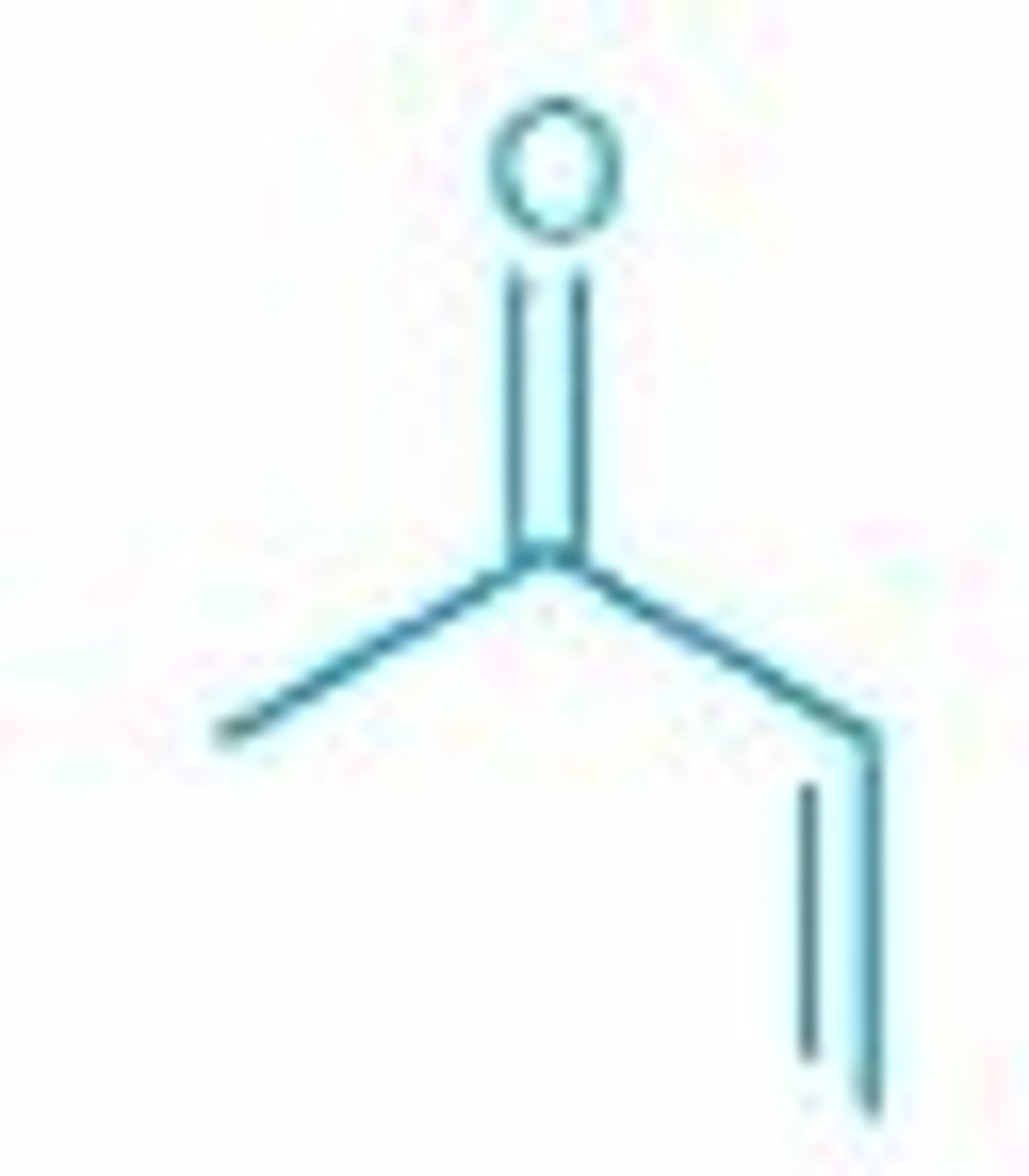
Name this compound
3-butene-2-one
For the following carboxylic acids, what are the common names?
methanoic acid
ethanoic acid
propanoic acid
formic acid
acetic acid
propionic acid