Chapter 16, Growth Factor and Cytokine Signaling Pathways That Control Gene Expression
1/53
Earn XP
Description and Tags
bolded terms and pathways defined
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
54 Terms
Differentiation
A population of cells slowly acquiring discrete fates and becoming specialized mature cells
Processes like this control embryonic development of all metazoans
“Given the role of gene transcription in mediating these critical aspects of development, is should come as no surprise that altered activity of these pathways is responsible for many human diseases including cancer, diabetes, and immune-system disorders.”
special transcription factors
that alter expression of a set of target genes that promote the needed change in cell behavior
stimulate or inhibit transcription of specific genes by binding to other DNA regulatory elements, called cis-regulatory elements (CREs aka enhancers). They regulate assembly or function of the RNA polymerase complex and in doing so control the on/off state of the target genes.
each specific transcription factor has affinity for a unique DNA sequence and therefore only bind CREs that have these sequences in them
For each specific transcription factor there are hundreds of target genes in the genome with CREs they can bind
General transcription factors
are required to transcribe all genes. they associate with RNA polymerase on the promoter of all genes to initiate transcription.
epigenetic state
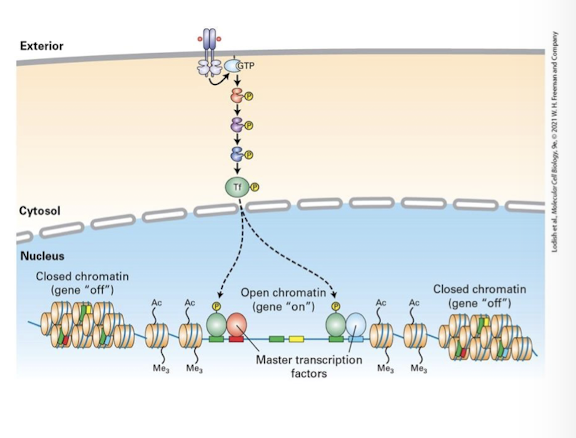
transduction pathways that activate the same specific transcription factor in different cell types may promote expression of different target genes
½ major reasons for this
epigenetic state:
not all regions of the genome are euchromatin (open chromatin) and therefore can be transcribed
Heterochromatin (closed chromatin) is tightly packed due to covalent modifications to histone proteins and the DNA itself
Regions of euchromatin vs heterochromatin vary from cell type to cell type - dependent on their developmental history
therefore, the same specific transcription factor activated in different cell types has different target genes it can bind and regulate.
Combinatorial control
transduction pathways that activate the same specific transcription factor in different cell types may promote expression of different target genes
½ major reasons for this
combinatorial control:
Most CREs are bound and regulated by multiple distinct specific transcription factors activated by different transduction pathways
A gene can be regulated only in cells that activate the right combination of specific transcription factors to binds its CRE
therefore, the same specific transcription factor activated in different cell types may regulate a different set of target genes based on the combination of other specific transcription factors also activate in that cell.
Protein-tyrosine kinase
family of kinases which phosphorylate specific tyrosine residues on target proteins
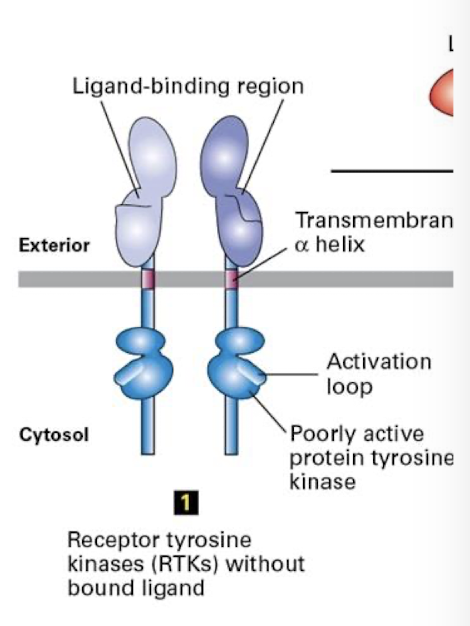
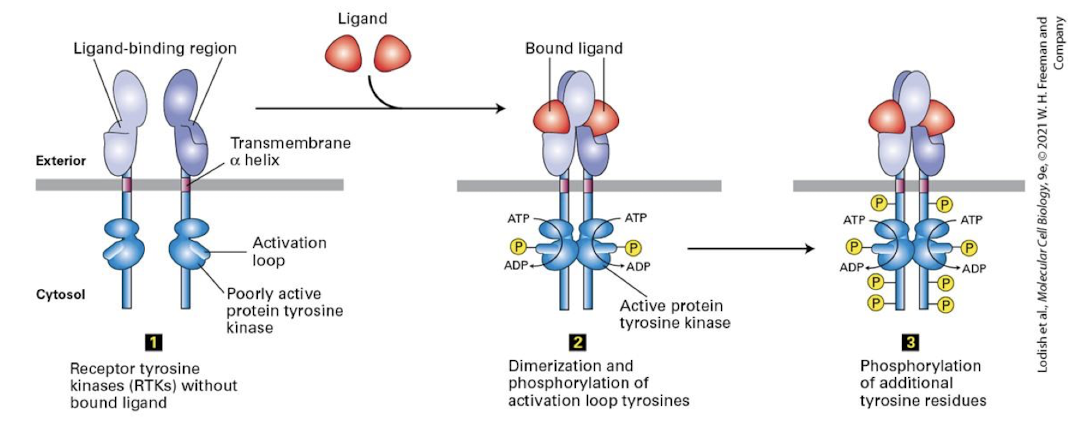
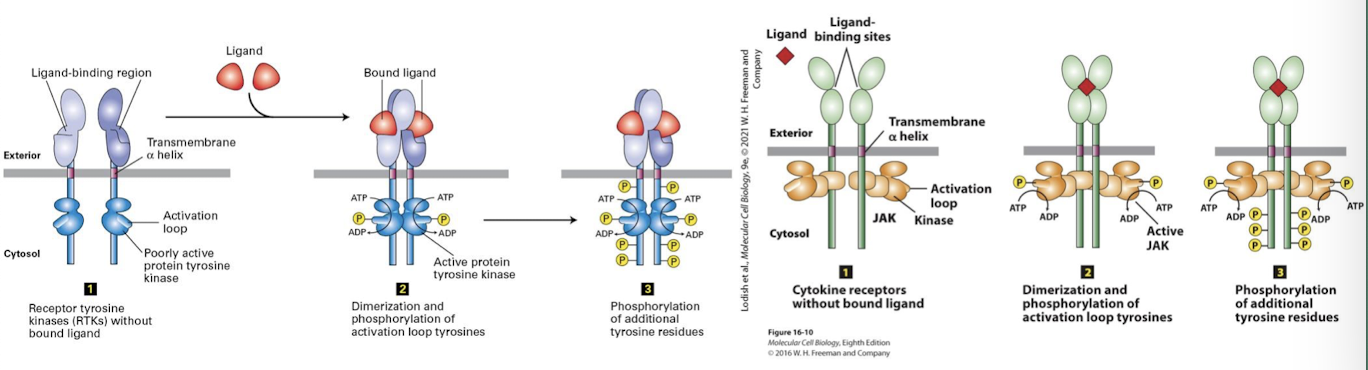
Receptor tyrosine kinase (RtK)
Cell surface reseptors that transduce signals from growth factors.
General features:
a single pass ⍺-helix transmembrane domain
an extracellular ligand binding domain
a cytosolic tyrosine kinase domain that is inactive when the receptor is not ligand bound
a cytosolic activation loop: switch that activates the kinase domain
requires ligand induced/ mediated/ dimerization
Growth factors
Ligands that stimulate cell growth and proliferation
An extracellular polypeptide molecule that binds to a cell-surface receptor, triggering an intracellular signaling pathway generally leading to cell proliferation
Activation Loop
A region in most protein-tyrosine kinases, containing a tyrosine residue that, when phosphorylated, increases kinase activity

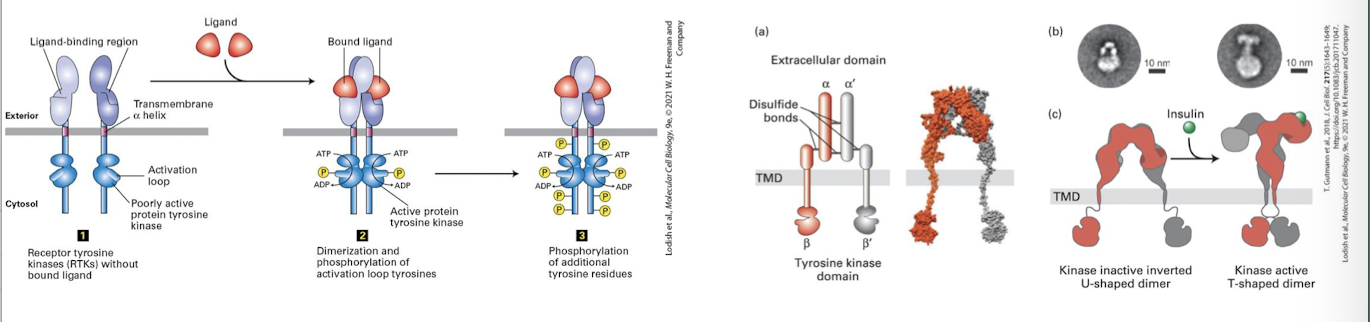
Ligand Induced Dimerization
Ligand binding induces RTK receptors to dimerize: A conformational change in the extracellular domain promotes molecular complementarity and dimerization.
bings two cytosolic domains together
basal kinase activity allows each RTK to auto-cross phosphorylate: they phosphorylate one another’s activation loop
this induces conformational change that triggers full kinase activity
homodimerization
between two identical proteins
heterodimerization
two similar but distinct proteins
auto-cross phosphorylate
basal kinase activity allows each RTK to auto-cross phosphorylate: they phosphorylate one another’s activation loop
Ligand mediated dimerization
Activated RTKs then auto-cross phosphorylate one another on multiple specific tyrosine residues within their cytosolic domains
these phosphotyrosines serce as docking sites for adapter proteins that transduce the signal to the cell interior
there are multiple phosphotyrosines on fully activated RTKs
Fibroblast
A common type of connective tissue cell that secretes collagen and other components of the extracellular matrix; migrates and proliferates during wound healing and in tissue culture
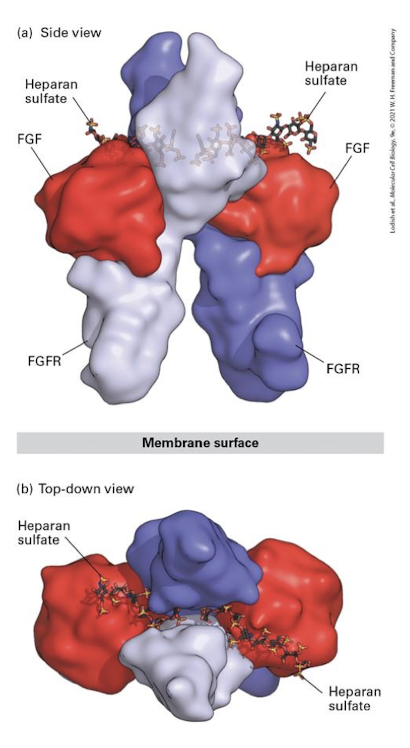
Fibroblast Growth Factor (FGF)
ligands induce FGFR dimerization
Fibroblast Growth Factor Receptor
Family of RTKs that transduce signals during development that regulate cell proliferation, migration, differentiation, and survival
major example of ECM involvement in cell-cell signaling:
FGF binding to FGFRs require association with the negatively charged heparan sulfate GAGs of ECM proteoglycans
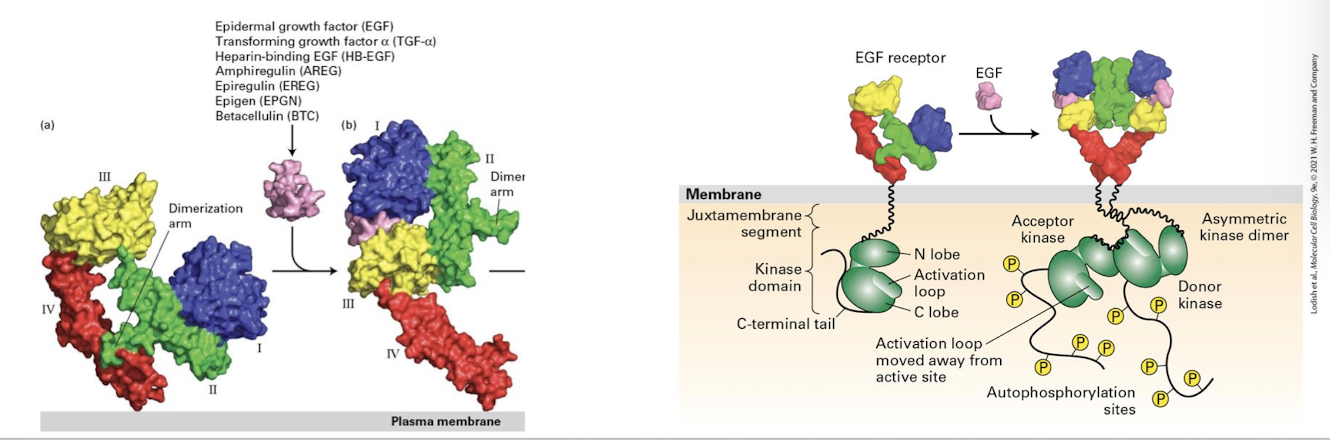
Epidermal Growth Factors (EGFs)
family of growth factors that promote cell proliferation
Human epidermal growth factor
HER 1-4
ligands binding to HERs promotes extension of dimerization and subsequent dimerization and activation via auto-cross phosphorylation
FGF and EGF and proliferation
FGF and EGF pathways promote proliferation
both sets of ligands and the pathways they activate/ promote proliferation
critical during embryogenesis and wound healing
mutations that inappropriately activate FGF and EGF pathways are major initiators of tumor formation and the onset of cancer
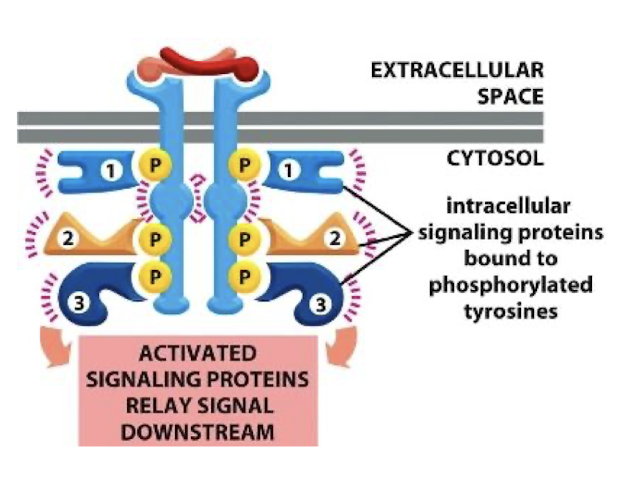
Adapter proteins
Adapter proteins physically link one protein to another protein by binding to both of them. Adapter proteins directly or indirectly (via additional adapters) connect cell-adhesion molecules or adhesion receptors to elements of the cytoskeleton or to intracellular signaling proteins
the phosphotyrosines on RTK dimers are bound by these transducting proteins
Docking domain
Adapter protein families share conserved docking domain that recognizes phosphotyrosines within specific amino acid sequences
this provides specificity that determines which pathways are regulated by each type of RTK
SH2 domain (Src Homology)
Most well-studied docking domain
there are over 100 human proteins that possess SH2 domains (the SH2 family)
Sub-family members have subtle differences in the SH2 domain that generates unique specificity for phosphotyrosines and therefore which receptor that SH2 proteins can bind
Attenuating the signal after RTK activation
Once signal transduction occurs the cell need to terminate signaling.
Receptor mediated endocytosis and lysosomal degradation prevents overstimulation of the process the RTK regulates
receptors are mono-ubiquitinated promoting endocytosis and lysosomal degradation in multivesicular bodies
EX) acivated HER receptors are ubiquitinated by a specific E3-ubiquitin ligase called c-Cbl
loss of function of c-Clb prevents appropriate attenuation of HER receptor signaling and is a driver of several forms of cancer the protein’s acronym name stands for Casitas B-Cell Lymphoma
Ras/MAPK signal transduction pathway
one of the most common cell proliferation pathways
almost all RTKs activate the Ras/MAP kinase pathway
functions by activating a MAP kinase cascade
uses the small monomeric G-protein Ras as the effector switch (Ras= Rat Sarcoma virus)
Activated Ras stimulates a MAPK cascade that controls cell cycle progression, proliferation and/or differentiation
Health relevance
most human cancers have gain of function mutations in an RTK, Ras, or a kinase in the pathway
Cells with mutants Ras constantly divide because the pathway is always active, even through there is no growth factor activating a receptor
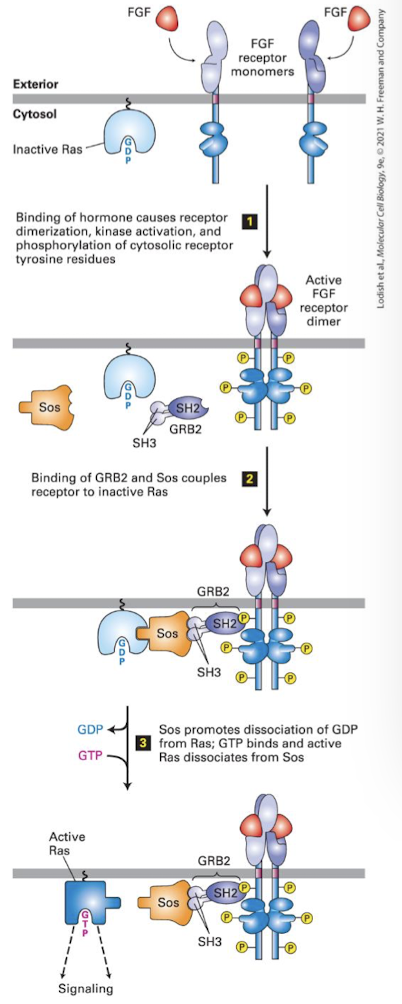
Canonical
Ligand (FGF, EGF, PDGF, etc) induces receptor dimerixation and auto cross-phosphorylation
Activated RTK bound by adapter protein Grb2 via its SH2 domain
Grb2 recruits the Ras GEF called SOS
SOS induces Ras to exchange GDP for GTP, activating it
Activated Ras recruits and acivates the first kinase in the cascade (the MEKK)
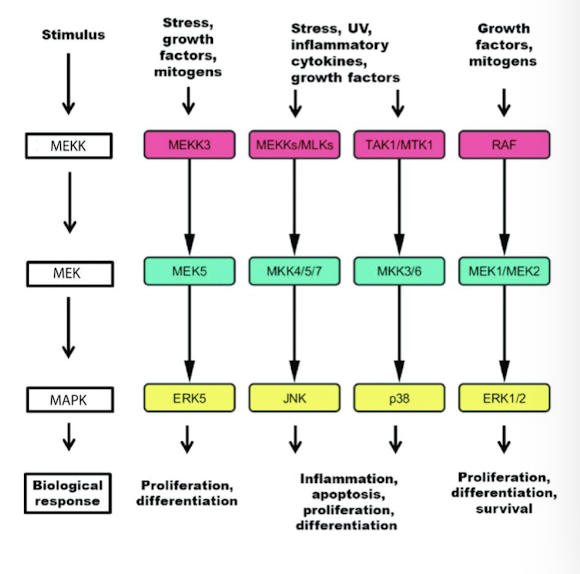
MAP kinase cascade
A series of three kinases that consecutively phosphorylate and activate the next kinase in the cascade
consist of three serine/ threonine kinases activated downstream of the receptor
MEKK
MEK
MAP Kinase
terminal MAPK phosphorylates and activates several cytoplasmic enzymes that perform an immediate response
it translocates into the nucleus and phosphorylates specific transcription factors that regulate expression of genes needed for the response.
they phosphorylate each other in that order
microtubule-associated protein (MAP)
any protein that binds to microtubules and regulates their stability
MAP kinase
mitogen activated protein kinase
any of a family of protein kinases that are activated in response to cell stimulation by many different growth factors and that mediate cellular responses by phosphrylating specific transcription factos and other target proteins
Ras protein
A monomeric member of the GTPase superfamily of switch proteins that is tethered to the plasma membrane by a lipid anchor and functions in intracellular signaling pathways; activated by ligand binding to receptor tyrosine kinases and some other cell-surface receptors
MEKK
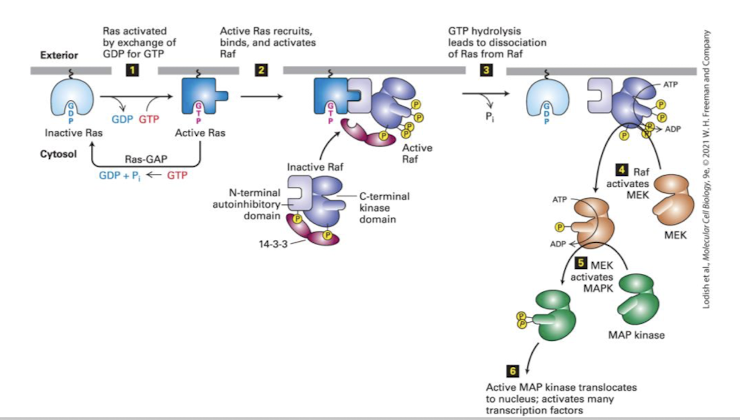
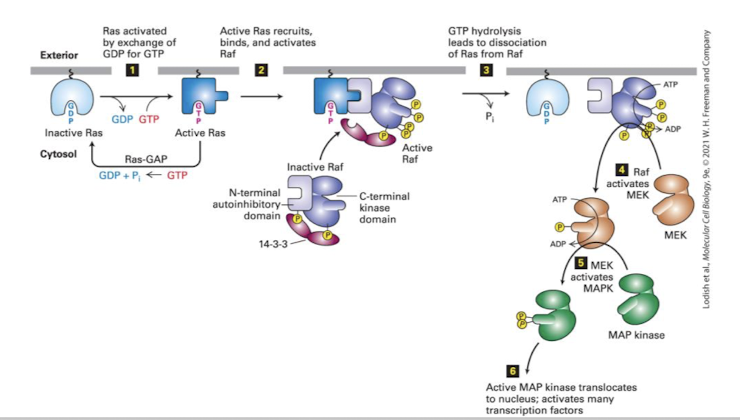
The Ras MAPK cascade
The MEKK is called Raf (Rapidly Amplifying Fibrosarcoma)
In unstimulated cells Raf is bound to an inhibitory protein called 14-3-3
Activated Ras binds and releases Raf from 14-3-3
Raf dimerizes and autocross phosphorylates
MEK
The Ras MAPK cascade
Activated Raf phosphorylates the next kinase, MEK (MAP Kinase of ERK Kinase)
Activated MEK phosphorylates the terminal MAPK, ERK (Extracellularly regulated kinase)
ERK dimerizes, phosphorylates cytoplasmic proteins and translocates into the nucleus
the pathway is silenced when Ras hydrolyzes GTP to GDP (often aided by a GAP)
MAPK
RTK activation as a mechanism for signal transduction: The Ras MAPK pathway
critical targets of MAPK:
the cytoplasmic kinase p90R5K
And the nuclear specific transcription factor Ternary Complex Factors (TCF)
p90R5K translocates into the nucleus and phosphorylates and activates the TCF co-factor Serum Response Factors (SRF)
cooperatively, TCF and SRF bind enhancers of early response genes- whose protein products push a cell from G1 of the cell cycle into S phase
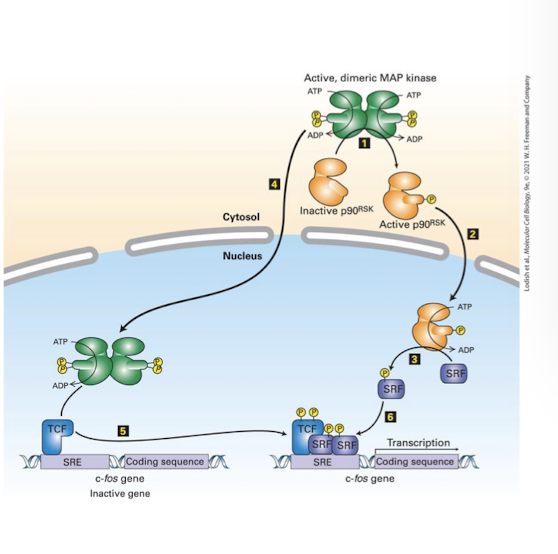
Scaffold proteins
Proteins that participate in intracellular signaling by binding several proteins in a signaling pathway and localizing them to a specific region of the cell
isolate distinct MAPK pathways in the same cell from one another
aids in specificity by tethering proteins of the pathway in specific spatial ortientations
ensures that activation of one MAPK pathway by a particular extracellular signal does not lead to inappropriate activation of other pathways containing shared components
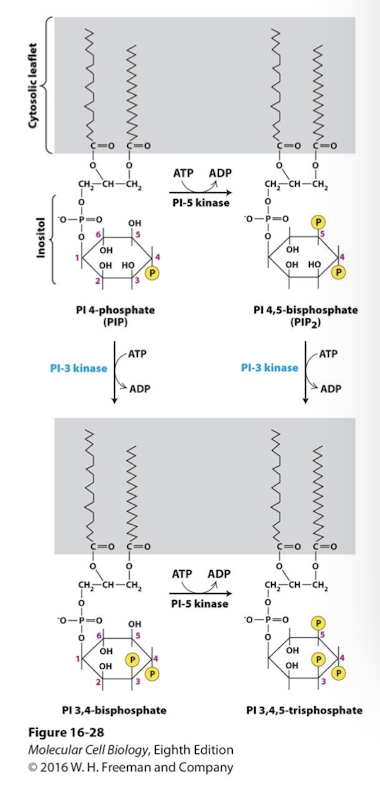
Phosphoinositide signal transduction pathways
PI kinases add phosphate groups to specific -OH groups of inositol carbons
some GPCR pathways activate Phospholipase C which cleaves PIP2 releasing IP3 and DAG as second messengers
some RTK pathways activate phospholipase C𝛾 which also cleaves PIP2 releasing IP3 and DAG
As with GPCR signaling IP3 promotes Ca2+ release from the ER and DAG activates Protein Kinase C family proteins
PI3 Kinase modify PIs to activate diverse downstream kinases
some RTKs phosphorylate and activate phosphoinositide 3-kinases
Active PI3K phosphorylates the 3’ carbon on one of two phosphoinositide substates
PI 4-phosphate
PI 4,5-disphosphate
yielding two distinct phosphatidylinositol 3-phosphates
PI 3,4 bisphosphate
PI 3,4,5 triphosphate
These phosphorylated PIs activate a kinase family that transduces the signal: Protein Kinase B family
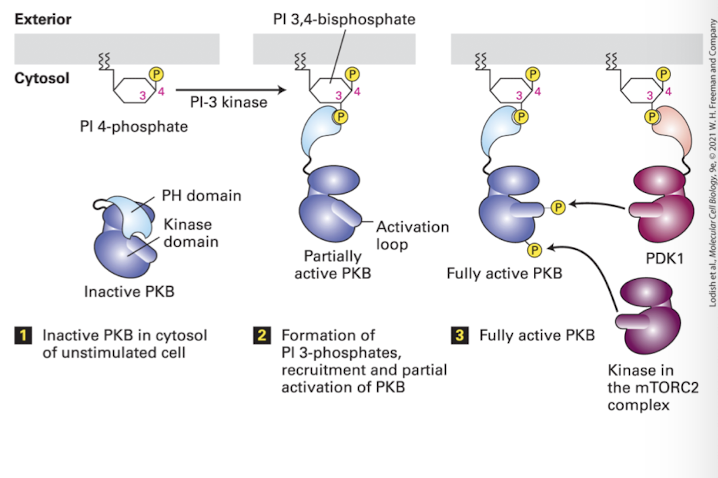
Protein Kinase B
Cytosolic enzyme that is recruited to the plasma membrane by signal-induced phosphoinositides and subsequently activated; also called Akt
Akt
A cytosolic serine/threonine kinase that is activated following binding to PI 3,4-bisphosphate and PI 3,4,5-trisphosphate; also called protein kinase B
Phosphoinositides
A group of membrane-bound lipids containing phosphorylated inositol derivates; some function as second messengers in several transduction pathways
Phosphoinositide 3-Kinase pathways (PI3K)
Protein Kinase B (Akt) binds PI 3,4-bisphosphate via a domain called the PH domain (plectin homology) which normally hides PKB’s kinase domain
Binding to PI3 exposes PKB Ser/Thr residues that are phosphorylated by another PI3,4-bP regulated kinase PDK1 (and a cytoplasmic PDK2, part of the MTOR complex)
ONce activated, PKB dissociates, and phosphorylates target proteins in a cell dependent manner. Principle celular functions are:
Anti-apoptosis (survival)
Pro-proliferation
Human Health Relevance:
Negative regulation: PKB activity is inhibited by the phosphatase PTEN, which not only removes activating phosphates from PKB, but also from PI 3,4-bP
PTEN is deleted in many types of advanced cancers which contributes to uncontrolled growth and evasion of the programmed cell death pathway
Cytokine receptors
Are non-covalently associated with cytoplasmic kinases what are activated following ligand binding. the term cytokine is interchangeable with growth factor.
Cytokine receptor signaling is also a major promoter of cell proliferation
Member of major class of cell-surface signaling receptors, including those for erythropoietin, growth hormone, interleukins, and interferons. Ligand binding leads to activation of cytosolic JAK kinases associated with the receptor, thereby initiating intracellular signaling pathways.
Erythropeietin (Epo)
A cytokine that triggers production of red blood cells by inducing the proliferation and differentiation of erythroid progenitor cells in the bone marrow.
Hormone released by our kidneys that triggers production of red blood cells (erythrocytes). It induces proliferation and differentiation of erythroid progenitor cells from stem cells in the bone marrow.
Health Relevance:
Epo is often given to patients receiving cancer therapy that reduces production of red blood cells
Cytokine
Any of numerous small, secreted proteins (e.g., erythropoietin, G-CSF, interferons, interleukins) that bind to cell-surface receptors on blood and immune-system cells to trigger their differentiation or proliferation.
JAK/STAT Signaling Pathway
Example of cytokine receptor signaling
Cytokine receptors do not have kinase domains but are non-covalently associated with members of a tyrosine kinase family JAKs (Janus Kinase)
require ligand induced dimerization
JAKs then phosphorylate receptor tyrosines generating docking sites for SH2 domain containing adapter proteins
Like RTKs this allows for multiple pathways to be simultaneously activated
all JAKs activate STAT proteins
when STATs bind phosphotyrosine of activated receptor they are phosphorylated by the JAKs
Phosphorylated STATs dissociate from receptor, homodimerize which exposes their nuclear localization signal, promoting entry into nucleus and gene activation
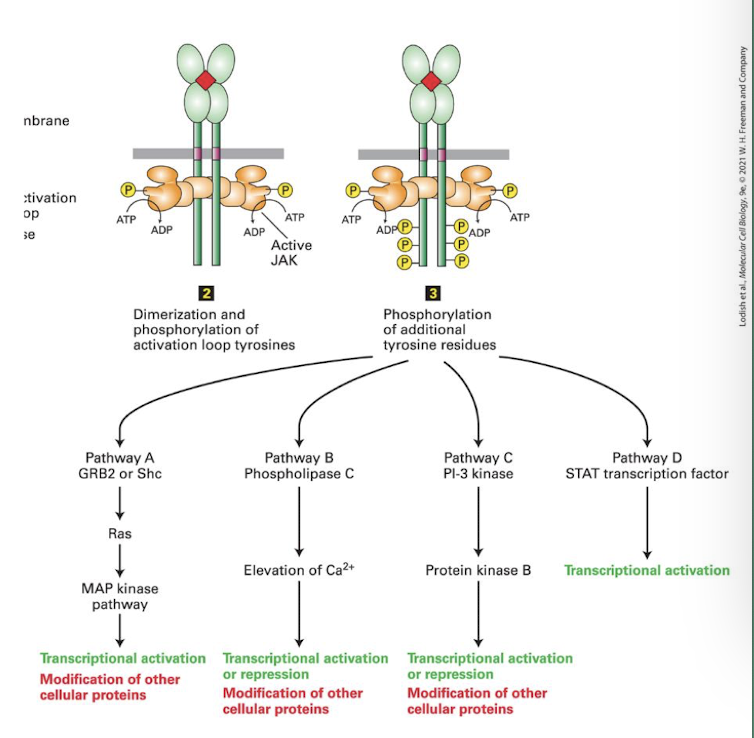
JAKs
Protein Tyrosine Kinases, like RTKs they auto-cross phosphorylate each other’s activation loop; enhancing their kinase activity.
STAT
A family of specific transcription factors that promote cell proliferation and inhibition of apoptosis
TGF-β/ Smad signaling pathway
Ligands form heterodimers or heterotrimers
Three required receptors:
RIII: binds all concentrates ligands at cell surface
They then transfer the ligands to RIII receptors, constitutively active Ser/Thr kinases that only phosphorylate class RI receptors (Constitutive means always on)
RII ligand binding alters conformation that allows them to bind RI receptors in a heterotetramer- 2 copies each of RII and RI
RII phosphorylates RI on conserved Ser/THr residues
RI kinase is active once phosphorylated
RI receptors phosphorylate Smad proteins: specifically, Smad2 or 3. This changes SMAD conformation and unmasks a nuclear localization signal
The Smads then bind Smad4 (its called a co-SMAD) and translocate into nucleus
In the nucleus the Smad complex binds enhancers and promotes transcription of target genes
Often collaboration with other transcription factos (like TFE3 in this example).
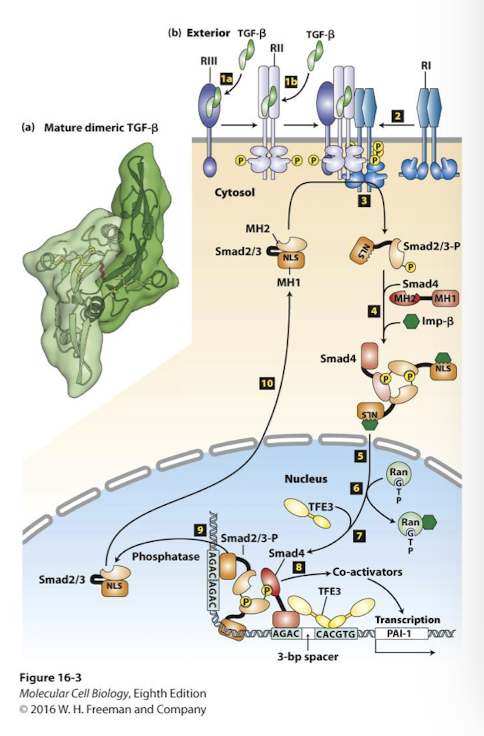
Smads
Class of transcription factors that are activated by phosphorylation following binding of members of the transforming growth factor beta (TGFβ) family of signaling molecules to their cell-surface receptors
Transforming Growth Factor Beta (TGFβ)
A family of secreted signaling proteins that are used in the development of most tissues in most or all animals. Members of the TGFβ family more often inhibit growth than stimulate it. Mutations in TGFβ signal transduction components are implicated in human cancer, including breast cancer.
Transforming growth factor. Large superfamily of related signaling ligands (~3 dozen encoded by human genome)
main role is to inhibit cell growth and proliferation
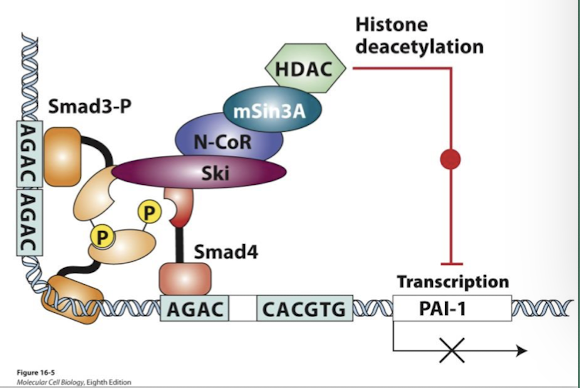
Ski-mediated down-regulation of Smad
Negative feedback loops regulate TGF-β/ Smad signaling
Ski is a TGF-β transcriptional target (Ski= Sloan kettering Institute)
Ski protein directly represses Smad function by preventing Smad4 from binding phosphorylated Smad3
Ski and a related protein Sno were identified as cancer-promoting oncoproteins. Their expression is elevated in many cancers.
The growth-inhibitory activity of TGF-β is blocked in cancer when these repressors are over-expressed
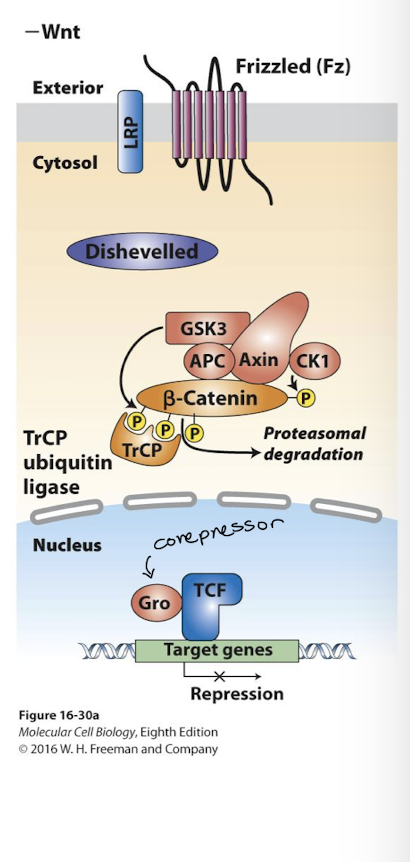
Wnt signaling pathway
an example of pathways controlled by ubiquitination and protein degradation
Wnt pathway promotes cell proliferation and differentiation. In absence of Wnt ligand:
nucleus: transcription factor called TCF (T Cell Factor) bind target gene enhancers with a transcriptional repressor called Groucho- target genes are silent.
cytoplasm: β-catenin is bound by the destruction complex (Axin, APC, and the kinases CK1 and GSK3)
GSK3 phosphorylates β-cateinin which targets β-catenin for ubiquitination and proteasomal degradation
Wnt ligand binds its receptor Frizzled (and a co-receptor)
THe activated receptor binds Axin of the Destruction Complex protein and the complex is disrupted
β-catenin is no longer degraded- it becomes stabilized
Free β-catenin translocates into nucleus and binds TCF, displacing repressor Groucho
Bound to β-catenin TCF activates expression of the same genes it was previously repressing.
Human Health Relevance:
90% of human colon cancers have hyperactive Wnt signaling. This primarily happens via loss of function mutations in a gene encoding one of the three Destruction Complex proteins APC
APC
adenomatous polyposis coli
Dominant loss of function mutations in the APC gene prevent activity of the destruction complex
Morphogen
A signaling molecule whose concentration determines the fate target-differentiating cells
Morphogen Function
A signaling protein that is secreted by a cell or group of cells and diffuses away creating a concentration gradient of signaling molecules.
Wnt signaling is a good example
Cells receiving the signal respond differently to different concentrations of the morphogen.
This is a critical process that drives the establishment of different cellular fates across a field of cells with similar developmental potential.
Hedgehod (Hh) signaling
Hedgehog signal proteins also act as morphogens on nearby cells promoting proliferation and differentiation
3 human family members:
Desert Hh
Indian Hh
Sonic Hh
Mutations in the Sonic Hh pathway are associated with a number of early congenital development defects.
Vertebrate Hedgehog (Hh) reception occurs only in primary cilia
In the absence of signal the Hh receptor Patched (Ptc) prevents a protein called Smoothened (Smo) from being inserted into the plasma membrane.
Smo remains in cytoplasm vesicles near the base of the primary cilium
A cytoplasmic protein complex phosphorylates a transcription factor Gli (glioblastoma) promoting its proteolytic cleavage into a transcriptional repressor- GliR
When Hh binds Ptc they are endocytosed and degraded by lysosomes
Smo enters the plasma membrane and is transported to tip of the primary cilium where Smo promotes dissociation of the Gli inhibiory complex
Gli is no longer cleaved Full length GliA (active) accumulates in the cytosol and translocates into the nucleus
GliA replases GliR and activates expression of the same genes GliR had been repressing.
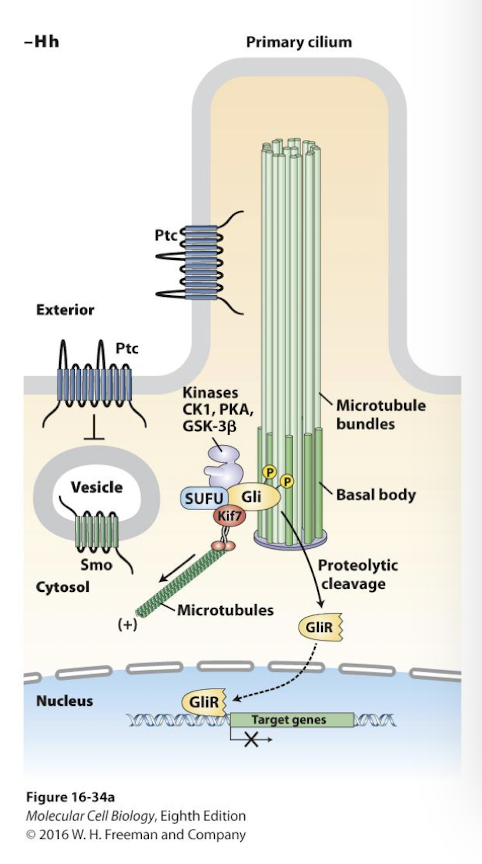
Primary Cilium
The single nonmotile cilium present on almost all vertebrate cells that serves as a sensory organelle to detect extracellular signals.