Water
1/8
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
9 Terms
Structure of water
Two hydrogens share electrons with an oxygen (two covalent bonds). Oxygen more electronegative so has a slight negative charge whilst hydrogen has a slight positive charge. This makes water polar.
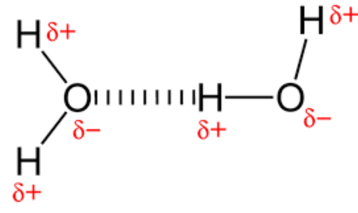
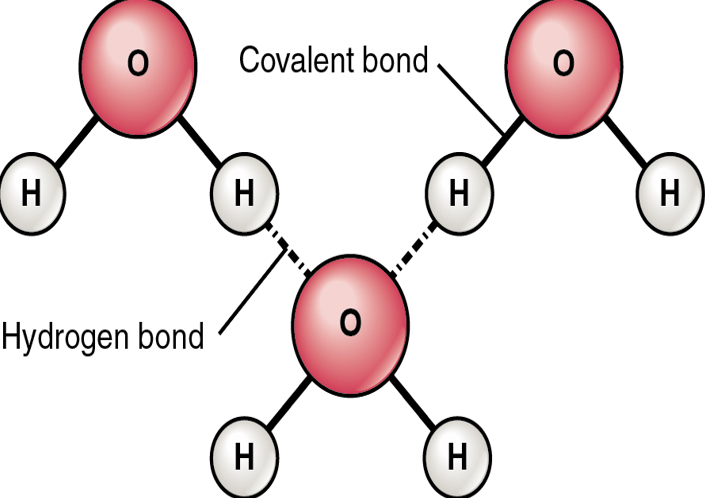
Hydrogen bonding
Hydrogen bonds are the weak bonds that form between the hydrogen and oxygen in another molecule.
Key properties of water
Good solvent
High specific heat capacity
High latent heat of vaporisation
High cohesion and surface tension
High adhesion
Density and freezing properties
Good solvent
Dissolves most charged and polar substances, which is important in chemical reactions and as a transport medium
High specific heat capacity
As hydrogen bond can absorb lots of energy it takes a lot of energy to heat up 1g by 1oc. This stops rapid temp rises and keeps temp fairly stable.
High latent heat of vaporisation
Lots of energy is required to break hydrogen bonds, so takes lots of energy to evaporate, making water great at cooling things.
High cohesion and surface tension
Very cohesive due to hydrogen bonds, helps water flow, so good for transporting substances. Cohesion generates surface tension
High adhesion
Water sticks to cell walls and xylem cells due to hydrogen bonding, so can be easily transported.
Density and freezing properties
Ice is less dense than water as below 4oc density of water decreases. This means that ice floats insulating water below it so water below does not freeze.