1.5 Tissue decalcification
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
24 Terms
Why is tissue decalcification necessary in histology?
Calcium deposits in tissues, such as bones or soft tissue tumors, make cutting sections difficult, so decalcification is needed to produce good quality slices.
What are the two main methods of tissue decalcification?
The two main methods are acid decalcification and chelation.
What is the chemical reaction for acid decalcification?
Ca₂(PO₄)₆(OH)₂ + 20H⁺ + 20NO₃⁻ → 10Ca²⁺ + 20NO₃⁻ + 6H₃PO₄ + 2H₂O
Which acids are commonly used for acid decalcification?
Nitric acid, hydrochloric acid, and formic acid are commonly used for acid decalcification.
What is the main disadvantage of prolonged acid decalcification?
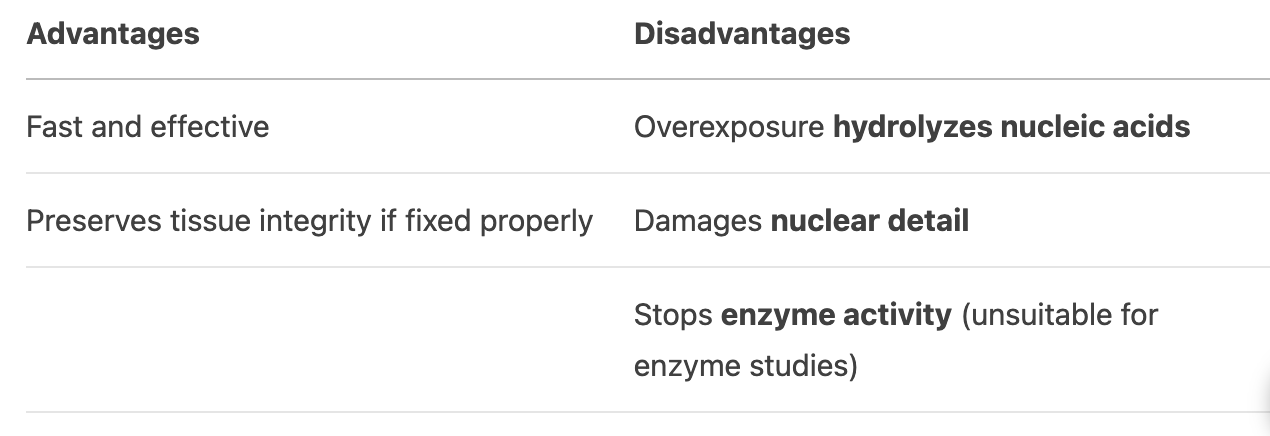
If used for too long, the acid can hydrolyze nucleic acids, damage nuclear detail, and stop enzyme activity.
How does fixation help in acid decalcification?
Proper fixation preserves tissue structure, reducing the risk of acid-induced damage.
How does chelation remove calcium deposits from tissues?
Chelating agents like EDTA bind ionized calcium and dissolve it from the hydroxyapatite crystals in the tissue.
What is the advantage of EDTA over acid decalcification?
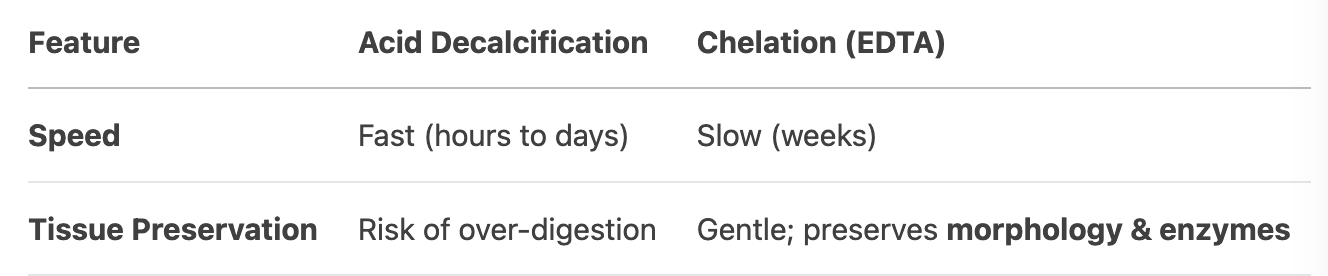
EDTA is gentle on tissue structures and preserves enzymes, unlike acids which may damage nuclear detail.
What is the major drawback of chelation compared to acid decalcification?
Chelation takes much longer than acid decalcification.
Why is decalcification necessary in histology?
Calcium deposits in bones or soft tissue tumors make sectioning difficult.
Decalcification removes calcium to produce high-quality tissue slices for microscopic examination.
What is the chemical reaction involved in acid decalcification?
Hydroxyapatite reacts with acid (H⁺) to form soluble calcium salts, phosphoric acid, and water:
Ca₁₀(PO₄)₆(OH)₂ + 20H⁺ → 10Ca²⁺ + 6H₃PO₄ + 2H₂O
Name three strong acids used in acid decalcification.
Nitric acid (HNO₃)
Hydrochloric acid (HCl)
Formic acid (HCOOH)
What are the advantages and disadvantages of acid decalcification?
How does EDTA work in chelation decalcification?
EDTA binds to ionized calcium (Ca²⁺) on hydroxyapatite crystals, forming soluble complexes that dissolve calcium.
Compare acid decalcification and chelation in terms of speed and tissue preservation.
Why is EDTA preferred for enzyme histochemistry studies?
EDTA preserves enzyme activity, unlike acids which denature proteins.
What happens if tissue is overexposed to acid during decalcification?
Nuclear details degrade, nucleic acids hydrolyze, and tissue becomes brittle.
When would you choose formic acid over nitric acid for decalcification?
Formic acid is milder than nitric acid, reducing risk of tissue damage while still being effective.
Why is fixation important before decalcification?
Fixation (e.g., formalin) stabilizes tissue proteins, preventing excessive damage during acid exposure.
What is a key disadvantage of using EDTA for routine decalcification?
Extremely slow process (may take weeks for dense bone).
How can you monitor decalcification progress?
Chemical tests (e.g., ammonium oxalate for calcium) or physical methods (needle probing).
Why is acid decalcification unsuitable for immunohistochemistry (IHC)?
Acids denature epitopes, compromising antigen-antibody binding in IHC.
What type of tissue is most challenging to decalcify?
Cortical bone (dense hydroxyapatite) requires longer decalcification than trabecular bone.
How does decalcification affect staining outcomes?
Over-decalcification leads to poor H&E staining (faded nuclei). Properly decalcified tissue retains crisp nuclear detail.