Properties of Water & States of Matter ~ Study Guide [Review 2024 ~ Team O]
1/43
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
44 Terms
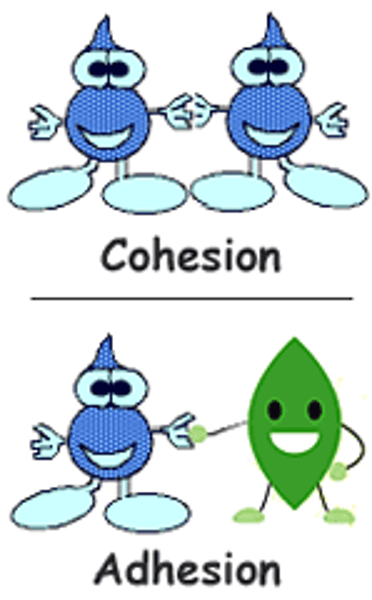
The tendency of water molecules to stick together is called ___________.
(example: water dome on the waxed paper)
cohesion

The tendency of water molecules to stick to other objects is called __________.
(example: water sticks to the side of a graduated cylinder)
adhesion

The ability of water to move upward through narrow tubes is called _________.
The water properties of cohesion and adhesion work with water property.
(example: blood moves through the capillaries in our body or the flower experiment)
capillary action

Water is known as the __________ __________ because it dissolves so many substances.
(examples: mixing salt or ice tea mix into a glass of water, making lemonade, making hot cocoa, etc.)
universal solvent
True or False: Water is made up of atoms bonded to form molecules.
True
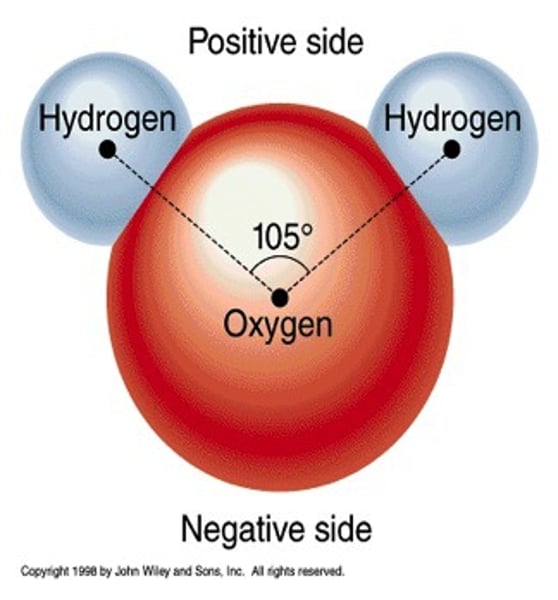
The chemical formula for water is _______. There are two (2) hydrogen atoms & one (1) oxygen atom that make up water.
H₂O
A molecule that has electrically charged areas is called a ____________ molecule. Water is a ________ molecule.
polar
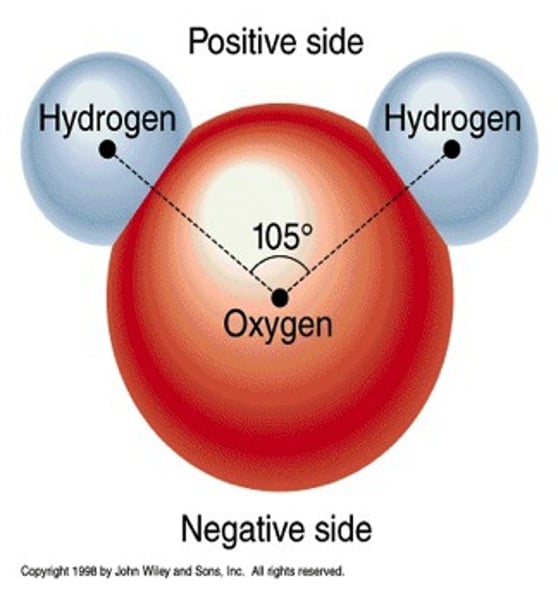
Each hydrogen atom has a slight __________ charge.
positive
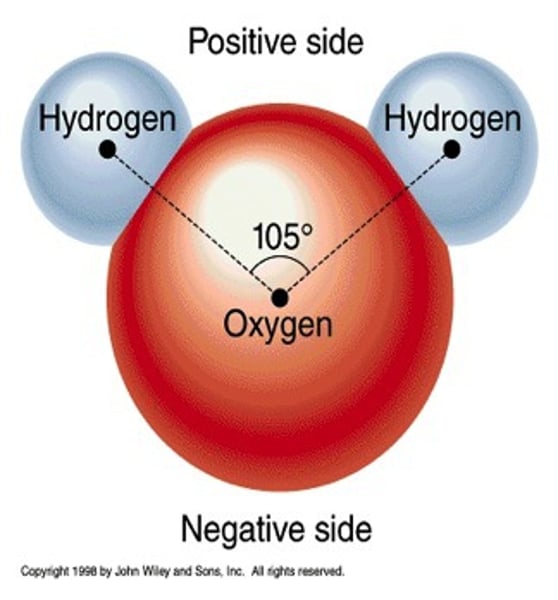
A oxygen molecule has a slight _________ charge.
negative

___________ __________ is the amount of heat it takes to raise the temperature of a substance by one degree Celsius.
specific heat
Because of specific heat, large bodies of water heat up/cool off more ___________ than nearby land [ex. sand].
slowly
In comparison to water's specific heat, land (sand) will heat up and cool down much ____________ than water.
quicker
The density of water is ___________.
1 g/cm3 or 1 g/mL
When an object’s density is less than water, the object will _______.
float
When the object’s density is greater than water, the object will ________.
sink

Ice is _________ dense than water and as a result, ice will float/stays on the surface.
less

As water temperature decreases, the weaker hydrogen bonds begin to hold the negatively-charged ________ atoms apart, forming a rigid crystal honeycomb structure we call ice.
Since ice has more volume, it has a lower density than liquid water. This causes ice to float in water!
oxygen

When mixing the blue salt water and the red freshwater, we learned freshwater is able to “float” on saltwater because saltwater is _______ dense.
more
![<p><span>The </span><u><span>substance that dissolves</span></u><span> [ex. sugar] to form a solution is called a __________.</span></p>](https://knowt-user-attachments.s3.amazonaws.com/f4453851-a9bb-48e6-97bf-77617cf24815.jpeg)
The substance that dissolves [ex. sugar] to form a solution is called a __________.
solute
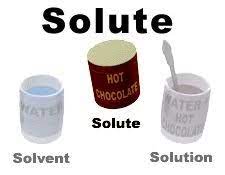
A mixture that forms when one substance dissolves another is called a _________.
solution
![<p><span>The substance that</span><u><span> does the dissolving </span></u><span>[ex. water] is called a __________.</span></p>](https://knowt-user-attachments.s3.amazonaws.com/5a883e44-48d2-47ef-bf56-d6a0db3d24e2.jpeg)
The substance that does the dissolving [ex. water] is called a __________.
solvent
Which type of change occurs when the starting state is a liquid and the ending state is a gas? This happens around 100 degrees Celsius.
evaporation [boiling]
Which type of change occurs when the starting state is a gas and the ending state is a liquid?
condensation
Evaporation occurs as water molecules __________ energy and it occurs at the surface of a liquid. Water is changing from a liquid to a gas.
An example of evaporation is air drying your wet hair.
absorb
________________ occurs as water molecules slow down changing from a gas to a liquid.
An example is when your breath clouds up a cold window.
Condensation
____________ is the only substance on Earth that commonly exists in all of its different states (solid, liquid, or gas).
Water
The percentage of the Earth's water that is fresh water is ______?
3%
The energy for the water cycle originally comes from the ____________.
sun
Water that falls to the Earth as rain, snow, sleet , or hail is called ______________. Water is returned to the Earth's surface from the atmosphere.
precipitation
The four factors that affect the rate (speed) of ___________ are temperature, surface area, humidity, and wind speed.
evaporation
The rate of evaporation ______________ with an increase in temperature.
increases
The amount of water vapor present in the air is called humidity. The rate of evaporation ____________ with an increase in humidity.
decreases
The rate of evaporation ______________ with an increase in surface area.
increases
Evaporation increases with an ______________ in wind speed.
increase
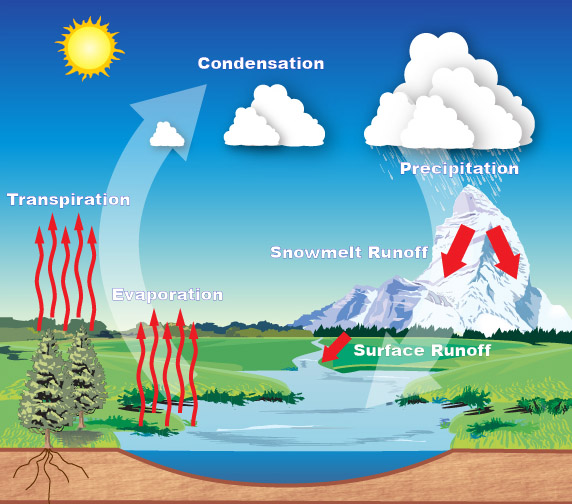
The process(es) of evaporation, precipitation, and condensation are involved with the _____________.
water cycle
____________ is the only substance on Earth that commonly exists in all of its different phases (solid, liquid, or gas).
Water
_____________ can exist in different states such as solid, liquid, and gas.
Matter
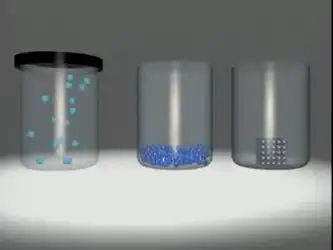
Which phase of matter holds its shape and has a fixed volume?
solid
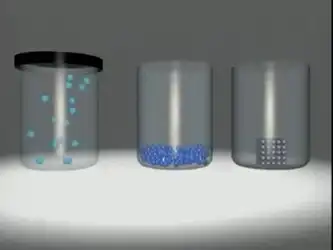
Which phase of matter has definite volume, but no definite shape?
A __________ will take the shape of a container.
liquid
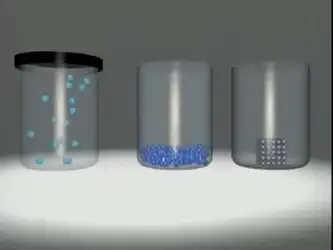
Which phase of matter has no definite volume and no definite shape? A __________ will expand to fill any container and it can also be compressed.
gas
Water in the _______ phase is called water vapor.

gas
What happens to water at 0°C (32°F)?

It freezes
Which phase of matter is found in stars?

plasma
When water freezes into ice, energy is __________ into the surroundings
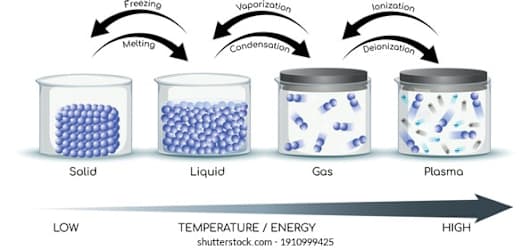
released