Chemistry EOT atomic structure
1/27
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
28 Terms
atom defenition and subatomic particle defenition
atom - smallest part of an element that can exist
subatomic particle - smaller fundamental components that make up atoms
Greeks and Dalton's Atomic Theory
tiny solid spheres that were small indivisible particles
Ex - more favourble way to say when you discover
you give evidence to prove the existence of
JJ Thompson - electron discovery - plum pudding model
in 1897 scientists (JJ Thompson) discovered that atoms contain tiny negatively charges particles - electrons
this then showed that atoms must have an internal structure and werent tiny indivisible spheres
thompson proposed the plum pudding model which was that an atom was a solid sphere of positive charge with negatively charged electrons embedded in it
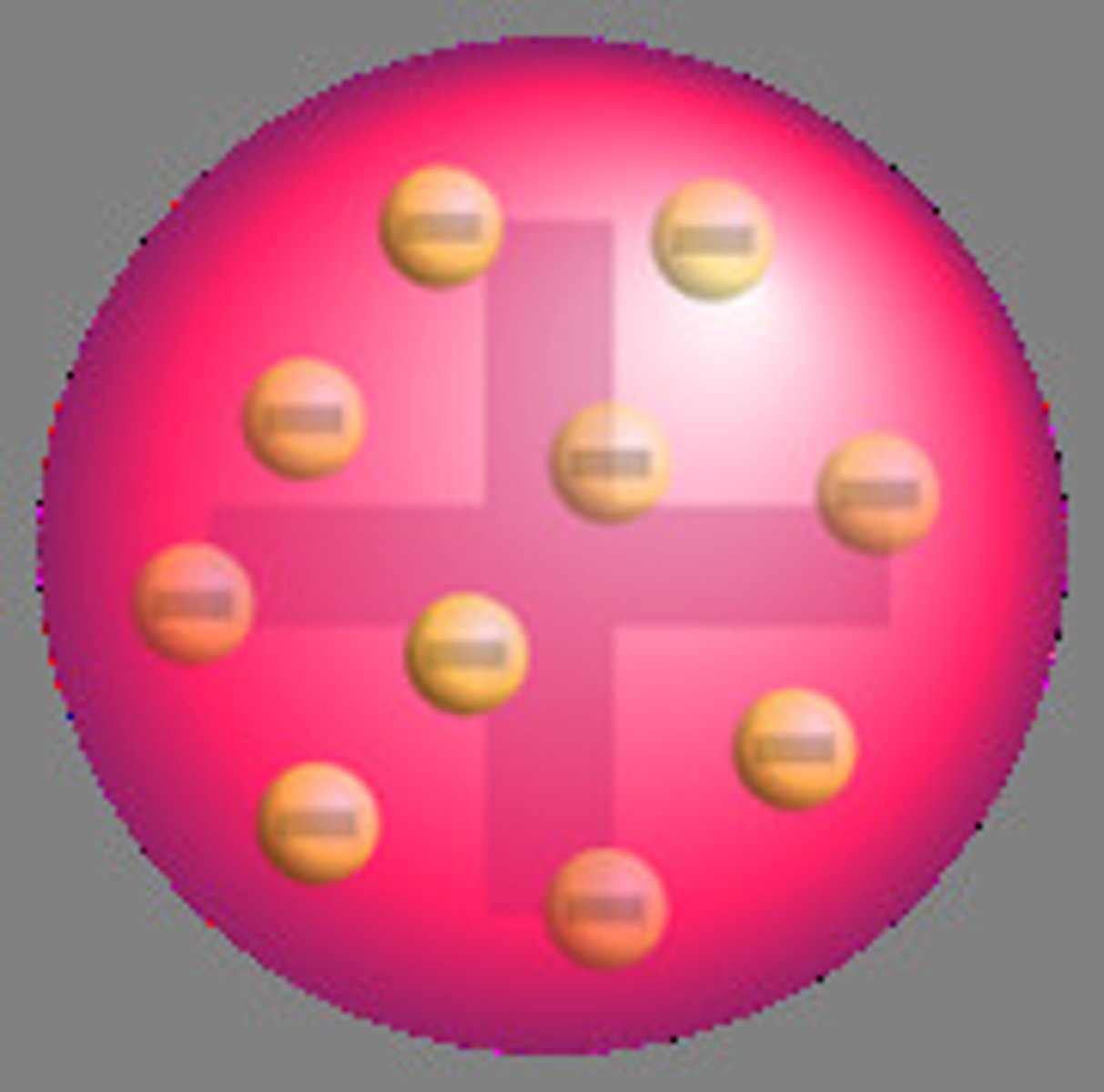
alpha scattering experiment - this was to see if the plum pudding model was right
took a piece of gold foil (gold because it can be hammered into very thin foil, just a few atoms thick)
then fired tiny + ⍺ particles (He2+) at the foil.
most of the particles went straight through the foil without
changing direction, sometimes particles were deflected repelled, (change direction)
and then some bounced back (reflection)
importance of the alpha scattering experiment
and importance of using and developing models
radically changed the way we think about atoms
New experimental evidence may lead to a scientific model being changed or replaced.
why the new evidence from the scattering experiment led to a
change in the atomic model
The fact that most of the ⍺ particles went straight through the foil showed that atoms were just empty space (so plum pudding model was wrong)
the fact that some ⍺ particles were deflected (repelled) showed that the centre of the atom must have a positive charge as + charge is needed to repel + charged ⍺ particle
the fact that some ⍺ particles bounced straight back showed that the centre of the atom, called the nucleus must contain a great deal of mass
differences between plum pudding model and nuclear model (V1) - nucleus and mass USE NEEM abbreviation
the pp model is a sphere of positive charge with negatively charged embedded electrons but in the nuclear model (all) the positive charge is held in (by) the nucleus
The mass in the plum pudding model is spread out through the sphere. (most of) the mass in the nuclear model is concentrated in the nucleus.
differences between plum pudding model and nuclear model (V1) - electrons empty space and modern model
in the plum pudding model, electrons are embedded but in the nuclear model the electrons and nucleus are separate (electrons are in orbits)
because the electrons in nuclear model are in orbits around the nucleus in the nuclear model, most of the atom is empty space but the pp model has no empty space and is solid
nuclear model (modern) contains protons and neutrons whereas pp doesnt
describe the nuclear model V1
most of the atom is empty space in the centre there is the positive dense nucleus which contains most of the mass of the atom around the nucleus there are tinier negative electrons in orbits,
further discoveries modified nuclear model v1 to modern nuclear model
Niels Bohr proposed that electrons orbit the nucleus at specific distances rather than a general which we know as shells or energy levels (based on calculations he had carried out) - accepted as agreed with results of other scientists
several yrs later scientists found out that the positive charge (chadwick) in the nucleus is due to tiny positive particles - protons - number of protons determines amount of positive charge in the nucleus
about 20 yrs after the nuclear model was proposed, Chadwick discovered neutrons in the nucleus - the neutral charge was late to be discovered as it wouldnt attract or repel any charges
describe (modern) + final nuclear model
most of the atom is empty space in the centre there is the positive nucleus which contains most of the mass (containing protons and neutrons) outside the nucleus electrons orbit at specific energy levels .
order of stuff discovery
1. atom 2. electron 3. (+) nucleus 4. electron energy levels
5. proton 6. neutron
relative charges and masses of subatomic particles
proton - relative charge : 1 relative mass : 1 , found in nucleus centre of atom
neutron - relative charge : 0 relative mass : 1 , found in nucleus centre of atom
electron - relative charge : -1 relative mass : very small , found in electron shells / energy levels nucleus orbital
the overall charge of the nucleus Is ___________ ?
the overall charge of an atom is _____________ ?
nucleus positive as protons (1) + neutrons (0) = positive (1)
atoms is neutral as there must always be same number of protons to electrons for the atom to be stable (stable atoms are neutral) and neutrons are side pieces as they dont have any charge
to find how many of what subatomic particle is in periodic table by symbols
electron and proton : number on atomic proton number
neutrons : mass number - atomic proton number
mass no.
number of protons , neutrons and electrons
mass number is up and atomic proton number is down - mass number is always bigger
The mass of electrons are neglected because their relative mass is very small, (but you can work it out as protons = electrons)
isotopes
atoms of an element with the same number of protons (therefore) electrons with a different number of neutrons resulting in a different mass no.
Relative atomic mass (Ar)
the average mass number of an element from their isotopes weighted by the abundance of each isotope relative to 1/12 of Carbon 12
- higher abundance make the Ar closer to that mass number - periodic tables often round this
ion
atoms or groups of atoms which have an overall electrical charge charge because they have lost or gained electrons in their outer shell
(electrons are -) so positive ions have lost electrons whereas negative ions have gained electrons
Ar formula
Ar =
Sum of (isotope abundance (%) x isotope mass number)
÷
100
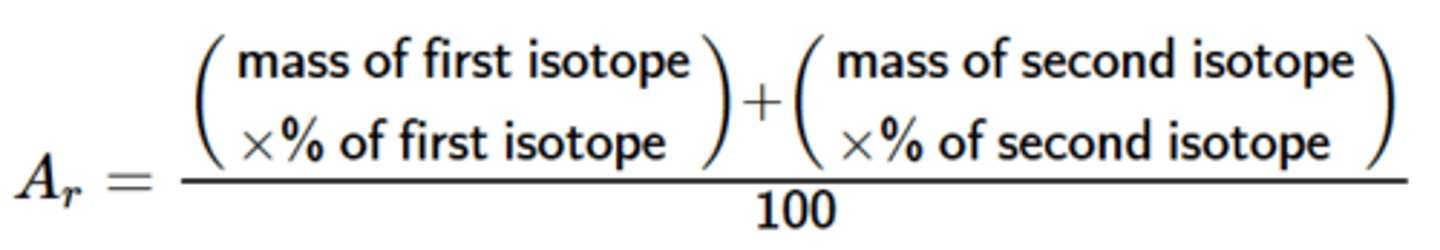
electron config facts
electrons occupy energy levels and the one closest to the nucleus is the lowest energy level - only certain number of electrons are allowed in each shell.
shell 1 . 2 elec. shell 2. 8 elec. shell 3. 8 elec. (max)
it can be written as formula as shell[1,2,3,4] e.g. calcium [2,8,8,2]
draw them with crosses and ofen exist in pairs
octet rule
the fact that noble gases are unreactive because they have full outer shells shows that in other elements their outer shell is not full which make them want to react to achieve a full outer shell.
ionic bonding - how ion charge links to group
reacting that takes place losing or gaining electrons forming ions and being attracted by strong electrostatic forces - between non metal and metals
because ions react to achieve a full outer shell so the number tells how many outer electrons need to be lost or gained to achienve a full outer shell
ion charge to group no
group : 1 2 3 4 5 6 7 0
ion charge : 1+ 2+ 3+ no ion. 3- 2- 1- no ion
metals form + ions (cations)
non metals form - ions (anions)
When naming ions u assume they are reacted so u always use IDE
ammonium sulfate nitrate carbonate hydroxide bicarbonate phosphate cyanide molecular ions
ammonium - NH4(+) Bicarbonate - HCO3(-)
sulfate - SO4(2-) phosphate - PO4(3-)
Nitrate - NO3(-) cyanide - CN(-)
carbonate - CO3(2-)
mendeleev
he developed his perioidic table in order of increasing atomic weight and not proton no. because protons were not discovered until the early 1917 and mendeleev developed in 1869
he often left gaps and changed the order in which elements were placed to make sure that the elements were placed in a group of similiar properties
Reactivity - tendency to form ions
We can see in the periodic table that other elements are more reactive than others, because they form ions easier, e.g. group 1 is easier because it only needs to lose 1 electron
and group 7 is also easier because it also only needs to gain 1 electron
compared to other groups