GenChem
5.0(1)
5.0(1)
Card Sorting
1/35
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
36 Terms
1
New cards
Aufbau Principle
* filling orbitals in order of increasing energy
* came from the word __***aufbauen***__ which means __***build***__
* came from the word __***aufbauen***__ which means __***build***__
2
New cards
Au (Gold)
does not follow aufbau principle
3
New cards
Diamagnetic
no unpaired electron ↓ (-1/2 )
4
New cards
Paramagnetic
has unpaired electron ↑ (1/2)
5
New cards
No. Types of Quantum Numbers
4
6
New cards
Principal quantum number
describes “shell” and “size” or orbitals
7
New cards
Azimuthal Quantum Number
describes the shape of the orbital
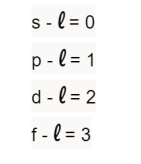
8
New cards
Pauli Exclusion Principle
* Filling the orbitals with two electrons in opposite spins
* like charges repel = unlike charges attract
* like charges repel = unlike charges attract
9
New cards
n (definition)
* main energy level of electron
* principal quantum number
* principal quantum number
10
New cards
s
sublevel in the first energy level
11
New cards
Spin Quantum Number (m*s*)
describes the spin of electron (1/2 or -1/2)
12
New cards
Electron Configuration
representation of the arrangement of electrons
13
New cards
Electric Structure of Atoms
series of energy levels that are possible for a bound electron to occupy
14
New cards
Shell
Energy level of atom/electron
15
New cards
Subshell (Sublevel)
tells the shape of orbital denoted as s, p, d, f
16
New cards
Energy levels
* n increases = energy increases
* \
* \
17
New cards
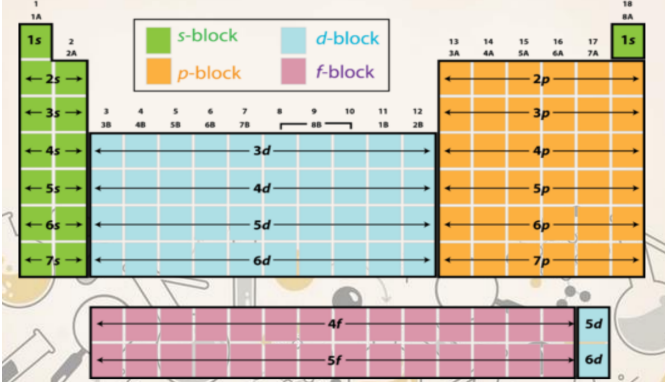
F orbitals (Fundamental)
* seven orbitals
* known as most diffused shape
* maximum of 14 electrons
* known as most diffused shape
* maximum of 14 electrons
18
New cards
P orbitals (Principal)
* 3 orbitals
* dumbbell shape
* maximum of 6 electrons
* dumbbell shape
* maximum of 6 electrons
19
New cards
s orbitals (sharp)
* 1 orbital
* spherical shape
* maximum of 2 electrons
* spherical shape
* maximum of 2 electrons
20
New cards
D orbitals (diffused)
* 5 orbitals
* maximum of 10 electrons
* maximum of 10 electrons
21
New cards
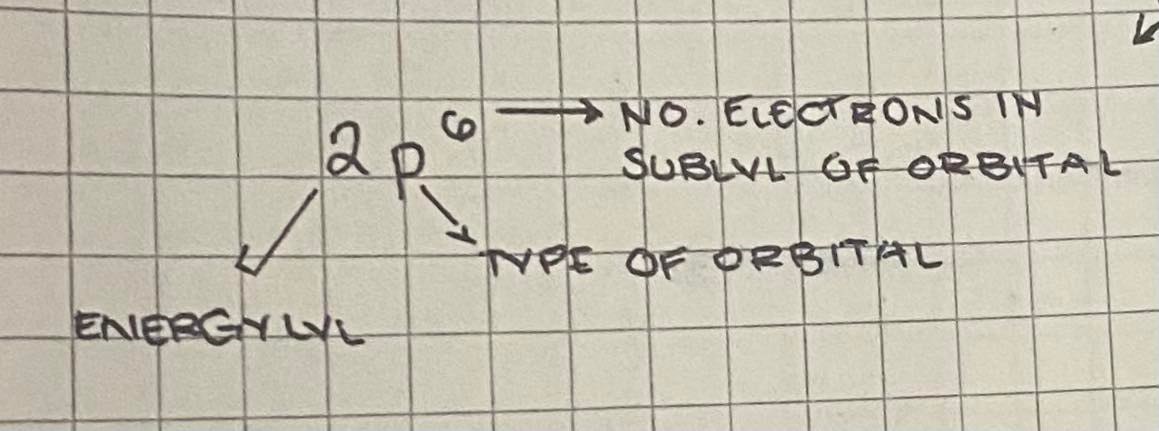
Electron Configuration
arrangement of electrons within their respective sublevels
22
New cards
s, p, d, f table
23
New cards
Noble gases
* inert gases
* all ends with p⁶ configuration (except helium)
* all ends with p⁶ configuration (except helium)
24
New cards
Duet rule
stable with only 2 electrons
25
New cards
Octet Rule
stable with 8 electrons
26
New cards
Noble Gas Configuration
use of noble gases in shortening the Electron Configuration
27
New cards
Orbital Diagram
reconstruct the electronic configuration.
28
New cards
Hund’s Rule of Multiplicity
All orbitals with the same energy must be filled up before pairing with another electron
29
New cards
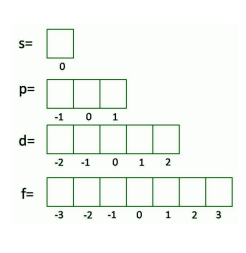
Magnetic Quantum Number
shows the orientation of the orbital in space
30
New cards
Chemical Bonding
basic fundamental that explains other concepts such as molecules and reactions
31
New cards
Lewis Dot Structure
representation of valence electrons
32
New cards
Octet Rule
atoms tend to lose, gain, or share electrons until they have achieved an outer shell that contains an octet of electrons
33
New cards
Ionic Bonds
electrostatic attraction between two oppositely charged ions cause by electrons transferring from one atom to another.
34
New cards
Properties of Ionic Compound
* High melting and boiling point
* Conducts electricity
* Solid at room temperature
* Hard and brittle
* Conducts electricity
* Solid at room temperature
* Hard and brittle
35
New cards
Outermost shell
Valence Electron
36
New cards
Paired dots
lone pairs