antigen presentation
1/50
Earn XP
Description and Tags
week 3 immunology
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
51 Terms
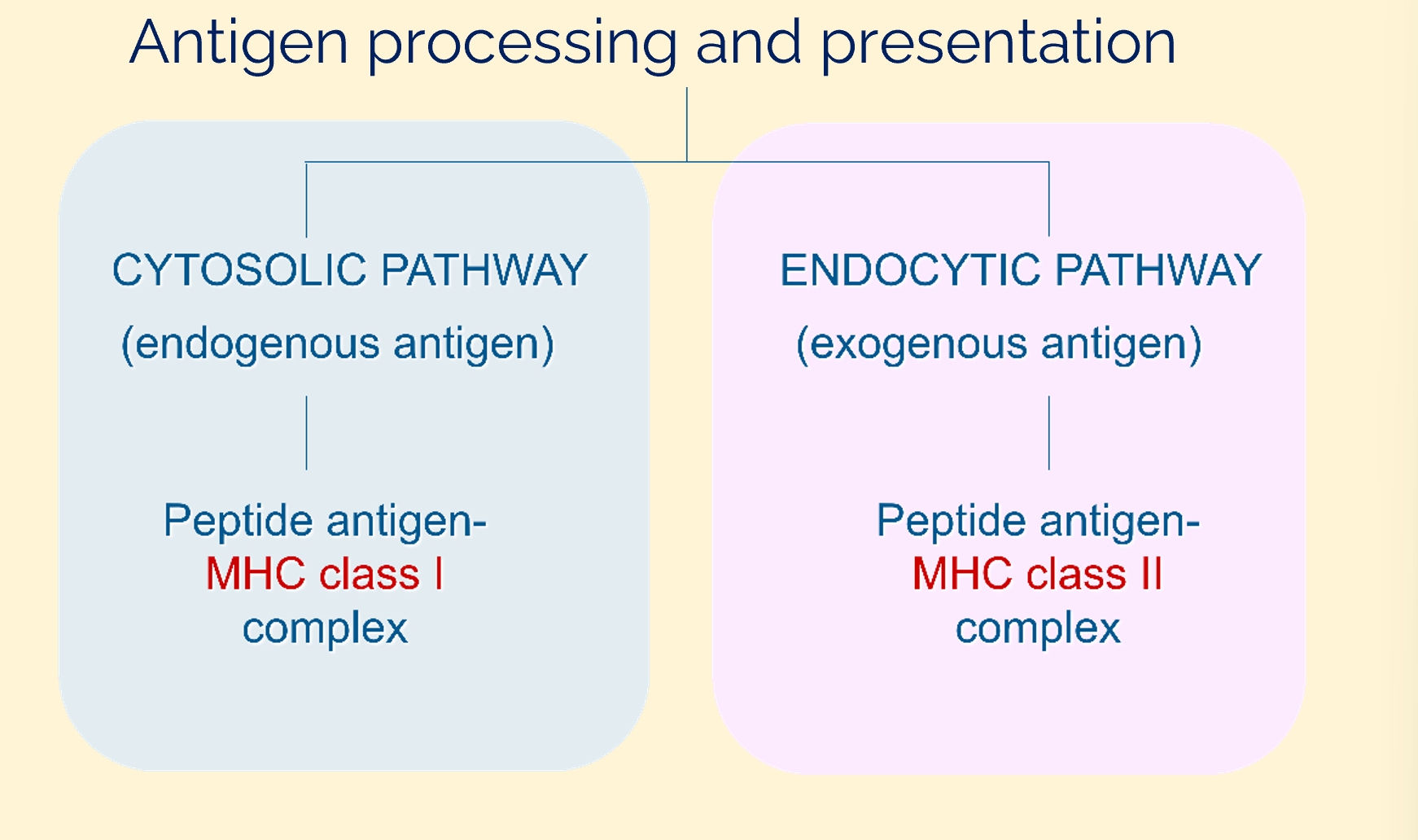
antigen processing is required to activate T cells
antigens must be processed and presented to immune cells
T cells recognise linear peptides derived from protein antigens
presentation is mediated by specialised protein molecules found on surface of APCs
MHC class I → CTLs (killer T cells)
MHC class II → Th cells
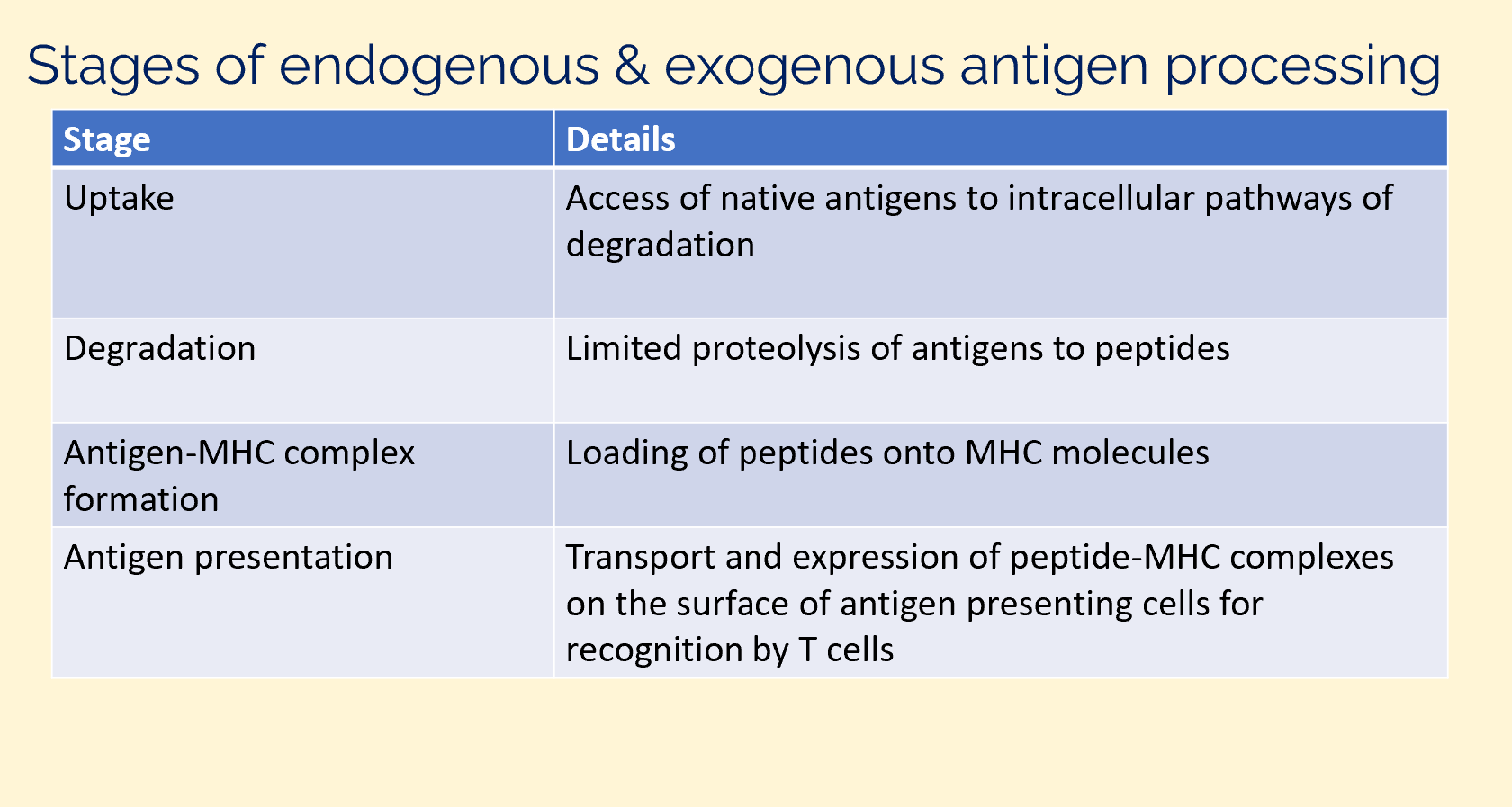
antigen peptides originate from
endogenous (intracellular) pathway
exogenous (extracellular) pathway
antigen processing and presentation
endogenous antigen processing: the endogenous antigen (a)
endogenous antigens can be cytosolic proteins but also virus particles
have to be processed to the appropriate size
8-10 amino acids optimum peptide length for MHC class I presentation
endogenous antigen processing (immuno)proteasome cleavage (b)
the proteasome cleaves polyubiquitinated proteins
peptides generated via proteasomal degradation
cytokine IFN γ increases 3 specialised catalytic proteosomal subunits:
β1i
β2i
β5i
these can replace homologous catalytic subunits in the housekeeping proteasome (immunoproteasome)
modifies cleavage specificity to tailor peptide production for class I binding (optimum peptide length)
endogenous antigens processing: peptide transport into ER and class I/peptide loading complex formation ( c )
peptides are transported into the endoplasmic reticulum (ER) by transporters
transporters associated with Antigen Processing (TAP1a and TAP2)- ATP dependent
TAP1/2 with calreticulin, tapasin and ERp57 form the peptide loading complex (PLC)
PLC loads the peptides into the class I MHC molecule
β-2 microglobulin (β2m): essential component of MHC class I molecule, required for expression of all MHC class I on the cell surface
endogenous antigens processing: release of class I/peptide from peptide loading complex (d)
following peptide loading, stable peptide MHC β2m complex (trimer) is released from PLC
endogenous antigens processing: transport through Golgi (e)
peptide- MHC β2m complex traverses the Golgi system
endogenous antigens processing: class I/peptide complex (f)
peptide- MHC β2m complex appears on surface ready for presentation to the T cell receptor (TCR)
exogenous antigen processing: exogenous protein uptake
exogenous protein is taken up by endocytosis
intracellular vesicles of dendritic cells, macrophages and B cells
in endosome, GILT (interferon γ - induced lysosomal thiol reductase) breaks any disulphide bonds in the engulfed proteins
progressive acidification in early endosomes’ proteolytic enzymes to cleave proteins into peptides
late endosomes contain lysosomal associated membrane proteins (LAMPS) which are implicated in enzyme targeting (autophagy)
exogenous antigen processing: MHC class II and li
MHC class II molecules assemble from α and β chains in the ER with the transmembrane invariant chain (li)
trimer recruits 3 more MHC class II molecules
li functions
ensures correct folding of nascent class II molecule
occupying MHC groove to inhibit spontaneous binding of peptides in the ER
combination of li with the αβ class II heterodimer inactivates a retention signal and allows transport to the Golgi
targeting motifs in the N terminal cytoplasmic region of li ensure delivery of the class II containing vesicle to the endocytic pathway
exogenous antigen processing: fusion
late endosomes fuse with the vacuole containing the class II-li complex forming the MHC class II enriches compartments (MIICs)
serine proteases cathepsin S and L and asparagine endopeptidase (AEP) degrade li, leaving only the part bound to MHC II groove
this part of li bound to MHC II is called CLass II-associated Invariant chain Peptide (CLIP)
exogenous antigen processing: removal of CLIP, antigen presentation
an MHC related dimeric molecule, DM, catalyses the removal of CLIP and keeps the groove open so peptides generated in the endosome can be inserted
initial peptide binding is determined by peptide conc and its on-rate but DM assists in removal of lower affinity peptides to allow their replacement by high affinity peptides
acidic pH required for exchange of peptides
complexes are transported to the cell surface for presentation to Th
MHC class II-like molecules HLA DM and HLA DO regulate the exchange of CLIP for other peptides
MHC II- CLIP can’t be released to cell surface unless another peptide replaces it
HLA-DM removes unstably bound peptides (peptide editing) to ensure stable peptide: MHC class II complexes that can survive long enough to stimulate CD4 T cells
HLA DO binds to HLA DM in same manner as MHC class II molecules (negative regulator)
MHC class II like molecules like HLA DM and HLA DO regulate exchange of CLIP for other peptides
in acidified endocytic compartment, HLA DO dissociates slowly from HLA DM
HLA DMA can then catalyse peptide editing for MHC class II molecules
IFN-γ ↑ HLA M expression but not of the HLA-DOβ chain
hence, IFN-γ produced by T cells and NK cells can ↑ expression of HLA DM and overcome inhibitory effects of HLA DO
advantages of peptide editing by DM
provides important safeguards
peptide: MHC complex must be stable at the cell surface
if peptides were to dissociate too readily, an infected cell could escape detection
if peptides could too easily be acquired from other cells, healthy cells might be mistakenly targeted for destruction
stages of endogenous and exogenous antigen processing
cross-presentation of antigens
25% of MHC class I present antigen of exogenous origin
up to 20% of MHC class II molecules present peptides from endogenous origin
non lysosomal antigen processing
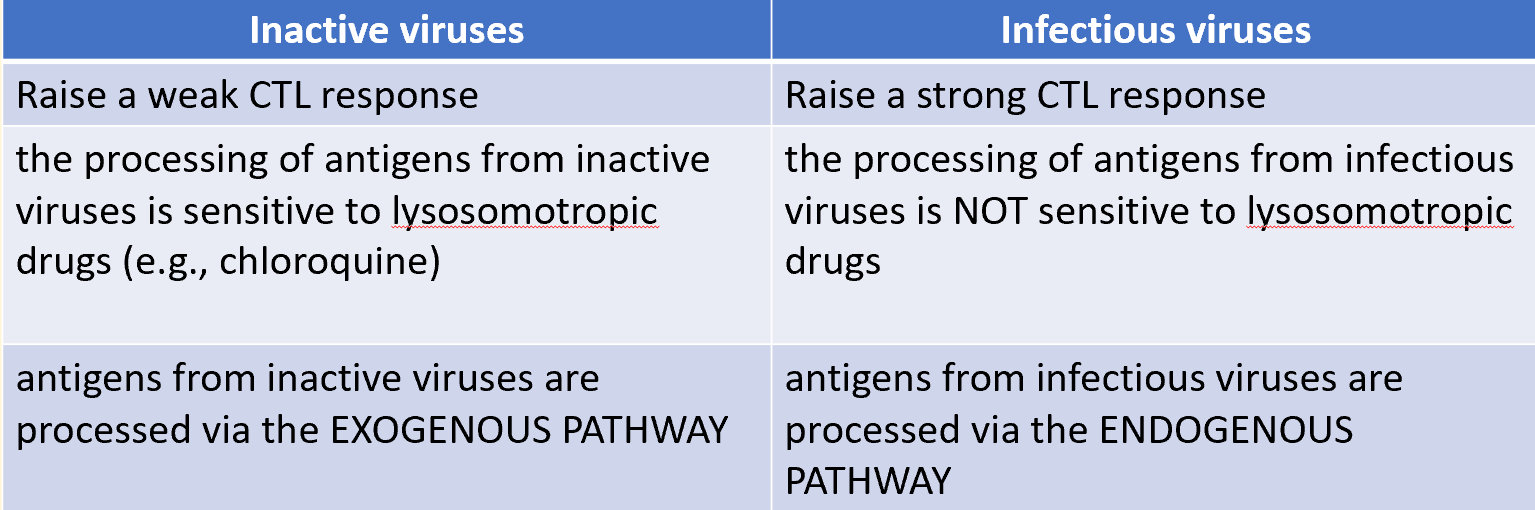
most CTL recognise antigens generated via a non-lysosomal pathway
protein synthesis is required for non-lysosomal antigen processing
inactive viruses vs infectious viruses
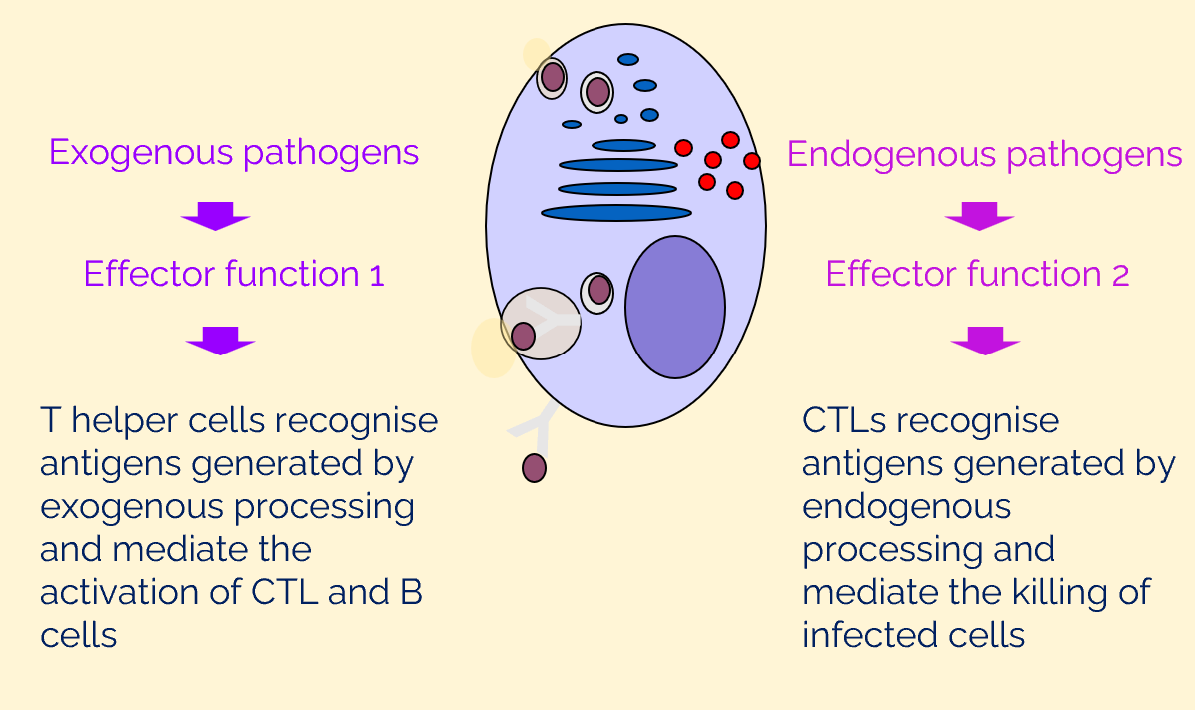
antigens generated by endogenous and exogenous antigen processing activate different effector functions
viruses have evolved mechanisms to hide from host immune system
MHC class I antigen presentation pathway is targeted by viral immune evasion proteins
inhibition of proteasome function
TAP-mediated peptide transport
chaperone facilitated peptide loading
transit of MHC class I from the ER
MHC class molecules
all cells present antigen on the surface via MHC
humans: HLA (human leukocyte antigen)
all nucleated cells display MHC class I molecules on their surface
professional APCs display MHC class I and class II
macrophages
dendritic cells
B cells
MHC class molecule structure
MHC class I: long heavy chain and β2 macroglobulin
MHC class II: α and β chain
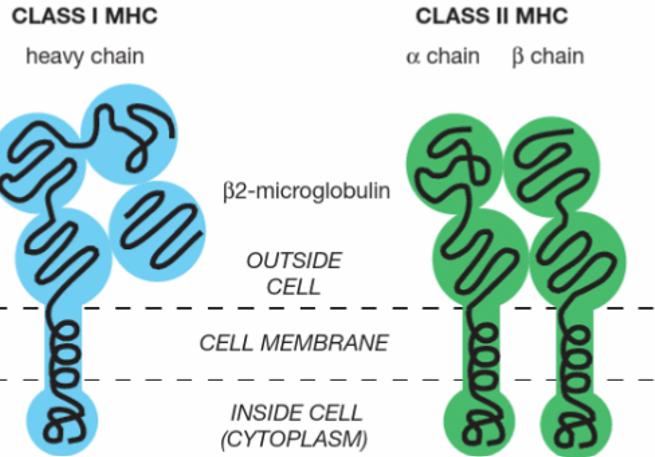
molecular structures of class I and class II MHC peptide complexes
the basic structures of MHC-I and MHC-II molecules are very similar but the way the peptide is bound and presented in binding cleft different between class I and class II
HLA locus
gene coding for MHC I and MHC II are located on chromosome 6
class I genes: A, B, C
each codes for a 3 domain peptide, associated with invariant β2microglobulin
class II genes: DP, DQ, DR
each codes for individual α and β chains that interact
HLA genes are highly variable (polymorphic) in sequence between individuals
antigen on MHC class I and T cell recognition
8-10 AA peptides noncovalently interact with the domains on the class I molecule and complementarity-determining regions (CDRs) on the T cell receptor (TCR) stabilised by the CD8 molecule
antigen on the MHC class II and T cell recognition
13-25 AA peptides interact with domains on the class II molecule within the peptide binding groove, allowing presentation to CD4+ Th cells
interactions with the TCR are stabilised by CD4 recognition of conserved regions on the class II molecule
T cell receptor (TCR)
transmembrane heterodimer
each T cell carries a TCR of only a single specificity
95% express α and β chains on their surface
5% express γ and δ on their surface
individual T cell can express either an αβ or γδ heterodimer, never both
TCR is always expressed with the CD3 complex, which is required for signal transduction
TCR diversity: α- and β-chain genes are composed of discrete segments that are joined by somatic recombination during T cell development
α chain (top part)
β chain (lower part)
α chain
Vα gene segment rearranges to a Jα gene segment to create a functional V-region exon
transcription and splicing of the VJα exon to Cα generates the mRNA that is translated to yield the TCR α-chain protein
β chain
variable domain is encoded in 3 gene segments
Vβ
Dβ
Jβ
rearrangement of these gene segments generates a functional VDJβ region exon that is transcribed and spliced to join Cβ
resulting mRNA is translated to yield the TCR β chain
TCR diversity is high
somatic rearrangement mechanism shared by immunoglobulin and TCR
overview of the number of human TCR gene segments and the sources of TCR diversity compared with those of immunoglobulins
TCR diversity is higher
TCR conc diversity in the 3rd hypervariable region (CDR3)
TCR antigen recognition site looks similar to that of the antigen-recognition site of an AB molecule
most variable parts of the T cell receptor interact with the peptide of a peptide: MHC complex
T cell activation
antigen induced activation of naive T cells initiates changes in:
morphology
metabolic activity (from predominantly oxidative phosphorylation to aerobic glycolysis)
progression into cell cycle
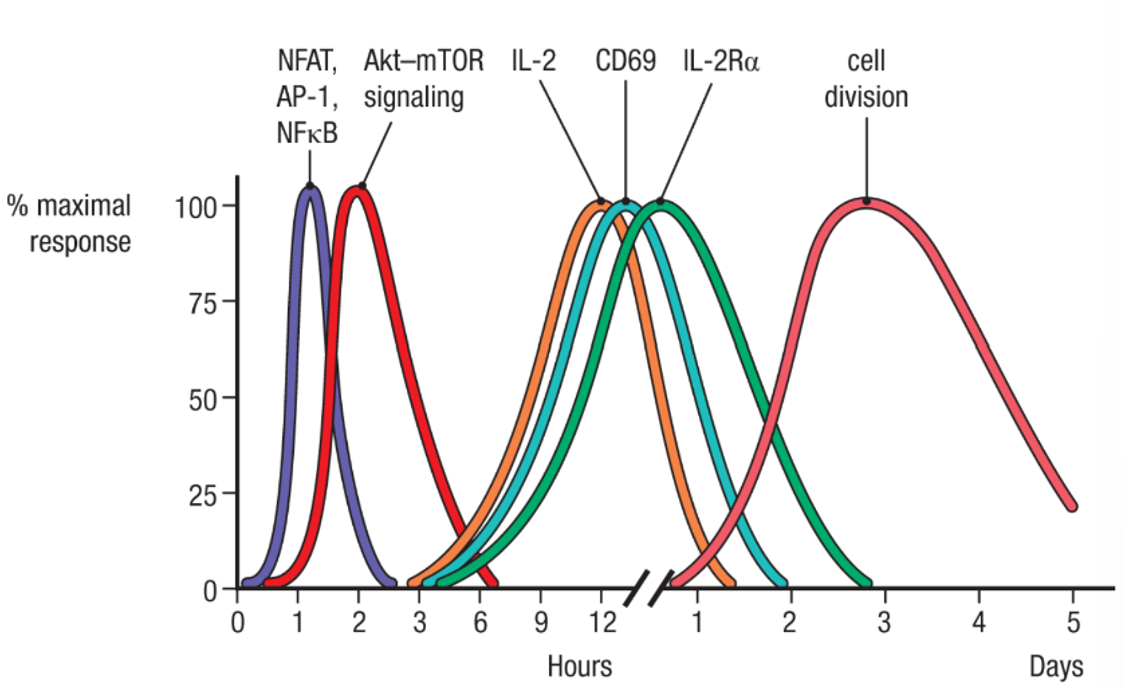
T cell activation: sequential waves of signalling protein activation, gene expression and cell cycle progression
transcription factors (NFAT, AP-1, NFκB) rapidly undergo changes in expression and/or post-translational modifications by signalling kinases upon activation by TCR and CD28 signalling
these factors then bind regulatory elements in many genes to activate their expression, including the genes encoding IL-2, CD69, and IL-2Rα
activation of the Akt–mTOR signalling cascade induces metabolic changes in the activated T cell to prepare it for transition from G0 to G1 as it enters the cell cycle
overall, leads to rapid cell division and clonal expansion
functional TCR complex
composed of antigen binding TCR α:β heterodimer
two ε
one δ
one γ
a homodimer of ζ (zeta)
all collectively called CD3
each CD3 chain has one immunoreceptor tyrosine based activation motif (ITAM) segment
each ζ chain has 3 ITAMs
transmembrane regions of each chain have unusual acidic or basic residues (indicated by plus and minus)
positively charged lysine of the α chain interacts with the 2 negatively charged aspartic acid of the CD3δ:ε dimer while positive arginine interacts with the negative charges of aspartic acid and glutamic acid in the CD3δ:ε dimer
co stimulatory receptors in T and B lymphocytes
naive lymphocytes require co-stimulatory receptors for activation
CD28 family of proteins (naive T cells)
TNF receptor superfamily/CD40 naive B cells
while naive T cells primarily utilise CD28 as the co-stimulatory receptor, naive B cells use the TNF receptor family
enhance the antigen receptor signals that induce transcription
factor activation and PI 3-kinase activation, thereby ensuring activation of the T or B cell
TCR signalling is initiated by tyrosine phosphorylation within ITAMs
CD3γ, δ, and ε r each contain one ITAM and each ζ contains 3, giving the T cell receptor a total of 10 ITAMs
each ITAM contains 2 tyrosine residues that become phosphorylated by specific protein tyrosine kinases upon ligand binding
when both tyrosines of the ITAM are phosphorylated, tandem SH2 domain containing proteins such as Syk or ZAP 70 are recruited
T cell activation- protein interactions
peptide: MHC complex must bind directly to the TCR
engagement of co-receptors (CD4 or CD8) with the TCR enhances ITAM phosphorylation
co-receptor-associated kinase Lck leads to phosphorylation (pink circles) of ITAMs in CD3γ, δ, and ε, and in the ζ chains
tyrosine kinase ZAP-70 binds to phosphorylated ITAMs through its SH2 domains enabling ZAP-70 to be phosphorylated and activated by Lck
ZAP-7P then phosphorylates other intracellular signalling molecules
roles of co-receptors CD4 and CD8
signal transduction
binding of the CD4 and CD8 molecules serves to transmit stimulatory signals to the T cells, signal transduction properties of both CD4 and CD8 are mediated through their cytoplasmic domains
stabilisation of TCR peptide: MHC interaction
additional binding of a co-receptor to the MHC molecule is thought to stabilise the interaction by increasing its duration
thereby providing time for an intracellular signal to be generated
T cell co stimulation enhances antigen receptor signalling pathways
activated Akt enhances cell survival and upregulates cell metabolism
recruitment of the kinase ltk to the membrane is critical for the full activation of PLC-γ
NFAT, AP-1 and NFκB stimulate expression cytokine IL-2, which is essential for promoting T-cell proliferation and differentiation into effector cells
MAPK pathway activates AP-1
calcium activates NFAT
protein kinase C activates NFκB
all three pathways are required to stimulate IL-2 transcription
gene activation requires binding of NFAT and AP-1 to a specific promoter element and additional AP-1 binding to another site
Oct1 is required for IL-2 transcription
Oct1 is constitutively bound to the promoter, hence not regulated by TCR or CD28 signalling
T cell activation leads to different cell subsets
naive CD8 T cells differentiate into cytotoxic T cells (often called cytotoxic T lymphocytes or CTLs) which are specialised for killing target cells bearing their cognate antigen
naive CD4 T cells differentiate into several types of Th or regulatory T effector cells
CD4 T cell subsets
TH1 cells
TH2 cells
TH17 cells
TFH cells
regulatory T cells
TH1 cells
produce cytokines, activating macrophages, (IFN-γ), enabling them to destroy intracellular microorganisms more efficiently
TH2 cells
produce cytokines that recruit and activate eosinophils (IL-5) as well as mast cells and basophils (IL-4) and promote enhanced barrier immunity at mucosal surfaces (IL-13) to eradicate helminths
T17 cells
secrete IL-17 family cytokines that induce local epithelial and stromal cells to produce chemokines that recruit neutrophils to sites of infection
produce IL-22 which activates epithelial cells at barrier sites to enhance barrier integrity and repair and produce antimicrobial peptides that kill bacteria
TFH cells
form cognate interactions with naive B cells through linked recognition of antigen and traffic to B cell follicles (where they produce germinal center response)
produce cytokines characteristic of other subsets that participate in type 1, 2 and 3 immune responses to influence isotype class switching
primarily produce IL-21, important for optimal production of high affinity, class switched ABs
regulatory T cells
suppress naive T cell responses and produces immune regulatory cytokines such as IL-10 and TGF-β which regulate response of effector T cells directly or via repression of pro-inflammatory cytokines