Topic 9 Redox Processes (electrochemistry) and Topic 19 (more) Electrolytic cells
1/47
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
48 Terms
what is an oxidation/reduction reaction (redox reaction)
any chemical relation that involves a shift of electron denisty from one atom to another
what are the old definitions for reduction and oxidation
oxidation: where a substance COMBINES wtih oxygen
ex. 2Mg + O2 → 2MgO
CH3OH + O2 → 2CO + 2H2O
reduction: REMOVAL of oxygen or addition of hydrogen
NiO + C → Ni + CO (smelting, Ni has been reduced, removed oxygen)
what are the new definitions for reduction and oxidation in terms of electron transfer
LEO GER
oxidation: loss of electrons
reduction: gain of electrons
what is a reducing agent
electron SUPPLYING substance during a redox reaction, LOSES electrions
causes other substance to be reduced
what is an oxidizing agent
electron ACCEPTING substance, gains electrons
causes otehr substance to be oxidized
what is an oxidation number
the charge an atom in a compound would have if the electrons in the bond belonged ENTIRELY to the more electronegative atom
how can oxidation numbers be used to identify the oxidation/reduction half reactions and the RA and OA
if theres a DECREASE in oxidation state PER ATOM, it is reduced, and acts as the OXIDIZING agent
if theres an INCREASE in oxidation state per atom, it is oxidized, and acts as a REDUCING agent
** if there is no change in oxidation numbers PER ATOM, it was not a redox reaction
what are the oxidation # rules for a monatomic ion and element
O# for monatomic ion is equal to the CHARGE of the ion
O# for element is ZERO
what are the oxidation # rules for alkali (group 1) and alkaline earth (group 2) family atoms in a compound
o# for alkali group 1 in a compound is +1
o# for alkali earth metals in a compound is +2
what are the oxidation # rules for oxygen and hydrogen in a compound
o# for oxygen in a compound is -2 ** except peroxides which are -1 (O2 2- = perioxide = -1)
o# for hydrogen is +1 ** except hydrides = -1 (when combined with a metal)
what are the rules for flourine or halogens
o# of flourine is ALWAYS -1 (because most electronegative)
o# of halogens are always -1 except when bonded with oxygen or a halogen higher up in the group (ClF, F = -1, Cl = +1)
what are the oxidation rules for compounds or a total reaction
all the TOTAL oxidation numbers for the elements making up a compound/ion must add up to the CHARGE of the compound
the NET change in TOTAL oxidation numbers for a reaction must be ZERO
what are the 7 steps to balancing redox reactions with acidic solutions
divide into half reactions
balance atoms OTHER than oxygen and hydrogen
balance O atoms using H2O
balance H atoms using H+
balance net charge in each reaction using e-
balance number of e- gained and lost by multiplying, then ADD two half reactions
cancel out anything that is the same on both sides
DONE!
what are the extra steps needed when balancing redox reactions with basic solutions
add the same number of OH - ions as there are H+ ions to BOTH sides of the equation
combined any OH- and H+ to form H2O
cancel out any H2O molecules you can
how is a redox table set up
on the top right, you have the strongest reducing agent RA, then weaker as you go down (metal elements)
on the bottom left, you have the stongest oxidizing agent OA, then weaker as you go up (metal ions)
what is the spontaneity rule using a redox table
it is spotaneous if… going from the OA to the RA, it is UP and to the right
it is NON-spontaneous if… going from the OA to the RA it is DOWN and to the right
what are the 5 steps to predicting redox reactions (if spontaneous or not)
identify each atom/ion as an oxidizing agent or reducing agent (write above or below)
choose the strongest OA, and write the equation for its reduction
choose the strongest RA, and write the equation for its oxidation
balance the number of electrons lost/gained by multiplying, then add the 2 half reactions
use the spontaneity rule for the reactant OA and RA, to see if it’s spontaneous
what are redox titrations
similar to acid/base titrations: used to determine the unknown concentrations of a substance, based on the redox reactions (electrons transferred instead of protons like with acids)
what is an indicator for in redox titrations
used to signal the equivalent point (equimolar), some redox reactions have their own colour change, thus additional indicator is not needed
when are redox titrations used/applications
food & beverage, pharmaceutical, water and environmental analysis
ex. vitamin c content of foods, detection of sulfur diocide in wine, iron conctent in supplements, concentration of chlorite in bleach
what is the biological oxygen demand (BOD)
defined as the amount of oxygen needed to decompose organic matter over a specific time at a specific temperature
used to measure the degree of pollution in water
what is the relationship between BOD, waste matter, dissolved oxygen, and pollution
high BOD = more waster = lower dissolved O2 levels = MORE POLLUTED
what is parts per million (how do you get it)
1 ppm = mass of solute in mg / volume of solution in dm³
whar are the reactions and final ratio that make up the winkler method
2Mn 2+ + O2 + 4OH- → 2 MnO2 + 2 H2O
MnO2 + 2I- + 4H+ → Mn 2+ + I2 + 2H2O
2S2O3 2- + I2 → 2 I- + S4O6 2-
OVERALL: 1 mol O2 → 4 moles S2O3 2-
what are the values of BOD for normal water, poor quality water, and sewage
normal = BOD < 1 ppm
poor = 20 ppm
sewage = 350 ppm
what is the winkler method
3 steps, used to measure dissolved oxygen in water
MnSO4 excess is added, to “fix” oxygen into MnO
iodide ions are oxidised by MnO, causing iodide normal atoms
I2 is titrated with Na2S2O3
OVERALL: for every 1 mol of O2, 4 mols of S2O3 2- are used (1:4)
what do voltaic / galvanic cells do
produce electricity through spontaneous redox reactions
cell potential is measured in volts with a voltmeter (1V = 1 J/C)
what are the parts of a voltaic cell
split into 2 parts connected by a salt bridge
each part has an electrode (metal) and its corresponding electrolyte (metal ion)
what are the functions of the salt bridge
physical separation between the cathode/ anode (between metal electrodes)
path for ions to flow to complete the circuit
reduces liquid junction potential (aka voltage reduction when 2 liquids touch)
what is the notation for a voltaic cell
(for zinc and copper)
includes the oxidation = anode, then reduction = cathode (each in diff half cells)
the metal for the anode is most reactive
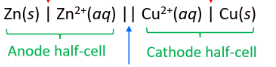
draw and label a voltaic battery with zinc and copper
should include…
1 molar zinc nitrate, zinc 2+ ions
1 molar copper nitrate, copper 2+ ions
ammonium nitrate salt bridge
electrolytes, solutions, voltmeter
show movement of the ions and electrons
LABEL: anode (neg because electron build up), oxidation, salt bridge, cations (towards the cathode), anions (towards the anode), cathode, reduction
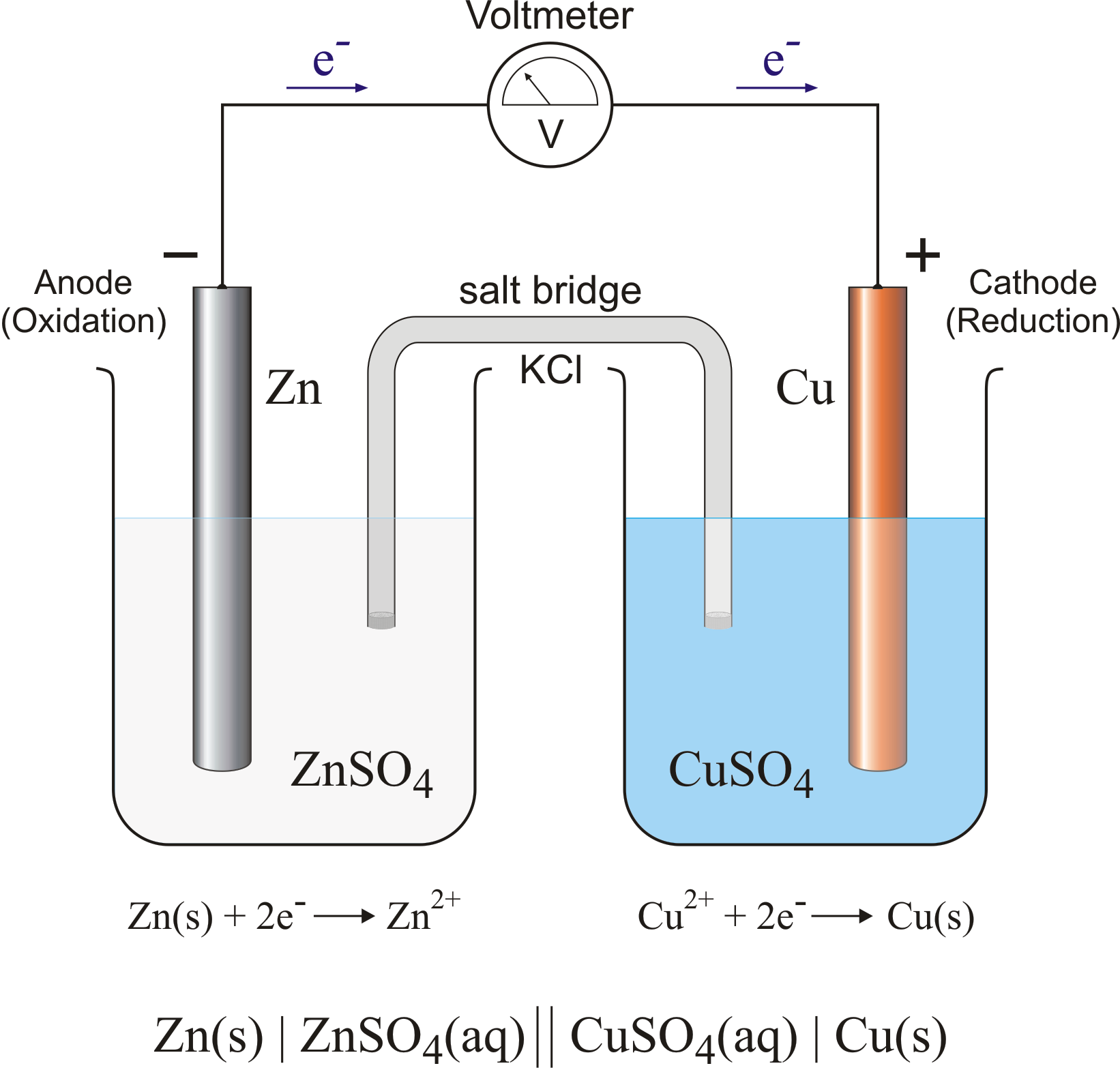
what is electrolysis
process where electrical energy is used to drive a non-spontaneous redox reaction
often used to purify metals, especially for metal ions that are poor oxidizing agents
what is an electrolytic cell
it has a single container, 2 electrodes (anode, cathode) a solution (electrolyte), and a battery/ source of electricity
what is a molten electrolytic cell
created by passing electricity through an ionic compound thats melted (LIQUID)
typically platinum electrodes are used as they are inert, don’t contribute to the overall redox reaction
explain how molten cells work (Pt | NaCl | Pt)
because of the battery, the electrons create the neg side = the cathode (reduction), the pos is the anode
at the cathode: Na + ions get electrons from the Pt strip, then form the solid Na
metal reduce at cathode
at the anode: Cl - ions lose electrons to the Pt strip, become Cl gas bubbles
non metal oxidized at anode
what are the similarities and differences between voltaic and electrolytic cells
similarities
both have electrodes
oxidation at anode, reduction at cathode
differences
voltaic: anode is neg because electrons build up because of oxidation (loss of electrons). electrolytic: CATHode is neg because of electrons coming from neg. end of battery
voltaic: not touching, 2 solutions, bridge. electrolytic: one solution, no bridge
voltaic: spontaneous. electrolytic: non-spont. needs energy
how does electrolysis of an aqueous solution work
passing current of electricity through an aquenous (dissolved in water) ionic compound / salt with an electrolytic cell
more difficult to predict the redox half reactions because water molecules can also be reduced/oxidized
describe the parts of the electrolysis of aqueous sodium chloride
Pt | NaCl (aq) | Pt
battery, with cathode neg (reduction), anode pos (oxidation), platinum electodes
one container with aqueous solution
how do you determine the cell potential of a cell
always E cell = E reduction + E oxidation
to find E oxidation, use DB, but must FLIP SIGN (because shown ones are for reduction)
for electrolysis of aqueous solutions, remember to write out all possible reduction reactions, pick the LEAST neg (most spontaneous) option, do the same for the oxidation
then multiple and add to create the overal reaction
what does the sign of the cell potential mean
negative is non-spont, (electrolytic)
pos is spontaneous (voltaic)
what are the factors that determine the possible redox reactions in an electrolytic aqueous cell
MAINLY relative reactivity (from DB)
less neg = more reactive/spont
but also depends on their concentrations
what are the applications of electrolytic cells
refining metals (copper, aluminum, mangesium)
electroplating (putting a metal on the surface of something)
what are the 3 main functions electroplating
make something more attractive (chrome plating car bumper)
prevent rusting of steel (galvanizing/ plating zinc onto iron)
jewelry (gold plating)
how does electroplating work/ what are the parts / describe the diagram
battery, neg end is cathode, connected to object you want plating ON
cathode is reduction, where positive metal ions go onto the object, to join with electrons
anode has material you want to use (silver bar). oxidation occurs, resulting in creation of metal ions
solution of aqueous ionic compound (ex. silver nitrate)
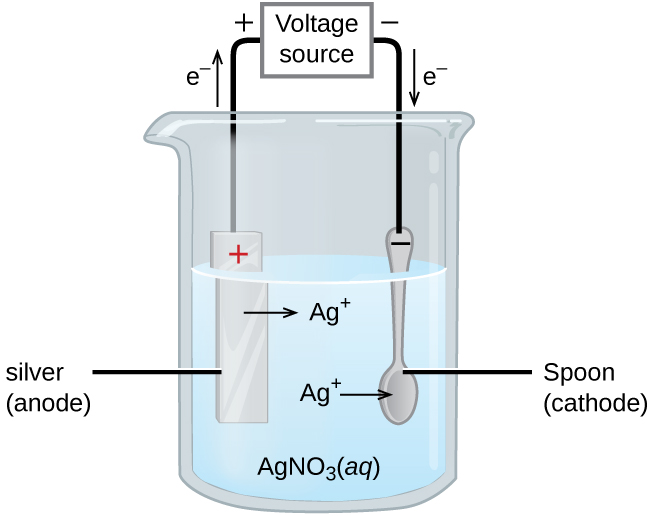
what are the factors that affect the amount of product formed on the electrodes during electrolysis
current
duration of electrolysis (longer = more)
charge of the ion
what is the important equation used to determine the time required to deposit a metal onto an object and the variables
n e- = I * t / F
n e- = # of moles of electrons
I = current in Amperes (1 A = 1 c/s)
t = time in seconds
what does the change in gibbs free energy mean in relation to spontaneity
negative Δ G = spontaneous (E cell +, voltaic)
positive Δ G = non-spont (E cell -, electrolytic)
what is the cell potential, gibbs free energy and type of reaction of a dead battery
cell potential = zero, gibbs free energy = zero,
reached equilibrium, no reaction