Dibartola Chapter 3: Disorders of Sodium and Water: Hypernatremia and Hyponatremia
1/202
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
203 Terms
What determines the volume of ECF?
Total body sodium content
What determines the osmolality and sodium concentrations of ECF?
Water balance
What is sensed for osmoregulation?
Plasma osmolality
What is sensed for volume regulation?
Effective circulating volume
What are the sensors in osmoregulation?
Hypothalamic osmoreceptors
What are the sensors in volume regulation?
Carotid sinus
Aortic arch
Glomerular afferent arterioles
Cardiac atria
Large pulmonary vessels
What are the effectors of osmoregulation?
Vasopressin
Thirst
What are the effectors of volume regulation?
Renin angiotensin-aldosterone system
Sympathetic nervous system
Atrial natriuretic peptide
“Pressure natriuresis”
Antidiuretic hormone
What is affected by osmoregulation?
Water excretion
Water intake
What is affected by volume regulation?
Urine sodium excretion
Osmolality
The concentration of osmotically active particles in a solution
Function only of the number of particles, not related to their molecular weight, size, shape or charge
Number of particles of solute per kilogram of solvent
Osmolarity
The number of particles of solute per liter of solution
Hyperosmotic
Osmolality is greater than that of the reference solution (often plasma)
Hyposmotic
Osmolality is less than that of the reference solution
Isosmotic
Osmolality identical to that of the reference solution
Calculated Plasma Osmolality
BUN and glucose are converted from mg/dL to mmol/L by the conversion factors 2.8 and 18
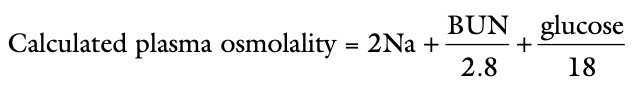
Measured osmolality should not exceed the calculated osmolality by more than ____?
10 mOsm/kg
If it does an abnormal osmolal gap is present
Occurs when an unmeasured solute is present in a large quantity in plasma (e.g. mannitol or metabolites of ethylene glycol) or when hyperlipemia or hyperproteinemia results in pseudohyponatremia
Specific Gravity
The ratio of the weight of a volume of liquid to the weight of an equal volume of distilled water
Depends on the number of particles present in the solution as well as their molecular weight
Effective Osmolality
A change in the concentration of permeant solutes (e.g. urea, ethanol) does not cause movement of water because these solutes are distributed equally throughout total body water (TBW)
A change in the concentration of impermeant solutes (e.g. glucose, sodium) does cause movement of water because such solutes do not readily cross cell membranes
Tonicity
The ability of a solution to initiate water movement
Dependent on the presence of impermeant solutes in the solution
May be thought of as effect osmolality
Hypertonic
Solution with a concentration of impermeant solutes greater than another solution that it is separated from by a semipermeable membrane
Hypotonic
Solution with a concentration of impermeant solutes less than that of another solution
Isotonic
Solution with a concentration of impermeant solutes equal to that of the reference solution
Estimate of Tonicity or Effective Osmolality
Posm = BUN/2.8
Diuresis
Urine flow that is greater than normal
Solute or Osmotic Diuresis
Increased urine flow caused by excessive amounts of nonreabsorbed solute within the renal tubules
e.g. polyuria associated with diabetes mellitus, administration of mannitol
During osmotic diuresis urine osmolality approaches plasma osmolality
Water Diuresis
Increased urine flow caused by decreased reabsorption of solute-free water in the collecting ducts
e.g. polyuria associated with psychogenic polydipsia or diabetes insipidus
During water diuresis, urine osmolality is less than plasma osmolality
Isosthenuria
Urine with an osmolality equal to that of plasma
Hyposthenuria
Urine with an osmolality less than that of plasma
Hypersthenuria or Baruria
Urine with an osmolality greater than that of plasma
Hypertonic Dehydration
Results from pure water loss and loss of hypotonic fluid, increases tonicity of remaining body fluid
Isotonic Dehydration
Results from loss of fluid with the same osmolality as that of ECF, no osmotic stimulus for water movement and the remaining body fluids are unchanged in tonicity
Hypotonic Dehydration
Results from loss of hypertonic fluid or loss of isotonic fluid with water replacement, remaining body fluids become hypotonic
Change in ECF Volume with Pure Water Loss
Small decrease
Change in Total Solute Concentration of ECF with Pure Water Loss
Small increase
Change in ICF Volume with Pure Water Loss
Small decrease
Change in Total Solute Concentration of ICF with Pure Water Loss
Small increase
Change in ECF Volume with Hypotonic Fluid Loss
Moderate decrease
Change in Total Solute Concentration of ECF with Hypotonic Fluid Loss
Very small increase
Change in ICF Volume with Hypotonic Fluid Loss
Very small decrease
Change in Total Solute Concentration of ICF with Hypotonic Fluid Loss
Very small increase
Change in ECF Volume with Isotonic Dehydration
Large decrease
Change in Total Solute Concentration with Isotonic Dehydration
Normal
Change in ICF Volume with Isotonic Dehdyration
Normal
Change in Total Solute Concentration of ICF with Isotonic Dehydration
Normal
Change in ECF Volume with Hypertonic Fluid Loss
Very large decrease
Chance in Total Solute Concentration of the ECF with Hypertonic Fluid Loss
Very small decrease
Change in the ICF Volume with Hypertonic Fluid Loss
Very small increase
Change in the Total Solute Concentration of the ICF with Hypertonic Fluid Loss
Very small decrease
Change in ECF Volume with Isotonic Fluid Loss with Water Replacement
Large decrease
Change in Total Solute Concentration of the ECF with Isotonic Fluid Loss with Water Replacement
Small decrease
Change in ICF Volume with Isotonic Fluid Loss with Water Replacement
Small increase
Change in Total Solute Concentration of the ICF with Isotonic Fluid Loss with Water Replacement
Small decrease
What is serum sodium concentration an indication of?
The amount of sodium relative to the amount of water in the ECF
Provides no direct information about total body sodium content
What does hypernatremia imply?
An increased serum sodium concentration (hypernatremia) implies hyperosmolality
Hypernatremia develops when water intake has been inadequate, when the lost fluid is hypotonic to ECF, or when an excessive amount of sodium has been ingested or administered parenterally
What does hyponatremia imply?
A decreased serum sodium (hyponatremia) usually, but not always, implies hyposmolality
Hyponatremia develops when the patient is unable to excrete ingested water or when urinary and insensible fluid losses have a combined osmolality greater than that of ingested or parenterally administered fluids
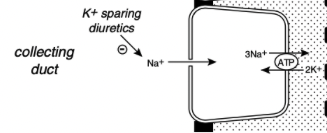
How is sodium removed from tubular cells in the kidney?
The metabolic energy for sodium transport in the kidney is required by Na+, K+ ATPase in the basolateral membranes of the tubular cells
This enzyme translocates sodium from the cytoplasm of the tubular cells to the peritubular interstitium and maintains a low intracellular concentration of sodium, which promotes sodium entry into the cell at the luminal surface
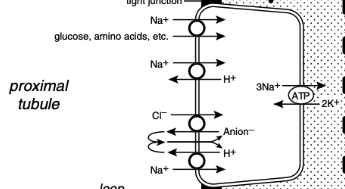
What percentage of the filtered load of sodium is reabsorbed in the proximal tubules
67%
Sodium Reabsorption in the Proximal Tubule
In the early proximal tubule, sodium crosses the luminal membrane by cotransport with glucose, amino acids, and phosphate and in exchange for H+ ions via the luminal Na+ - H+ antiporter
Reabsorption of water and sodium with HCO3- and other solutes in this segment of the nephron increases the Cl- concentration in tubular fluid and facilitates Cl- reabsorption later in the proximal tubule
In the late proximal tubule, sodium is reabsorbed primarily with Cl-
The luminal Na+ - H+ antiporter works in parallel with a luminal Cl- anion- antiporter and the net effect is NaCl reabsorption
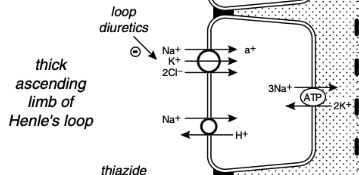
What % of the filtered load of sodium is reabsorbed in the loop of Henle
25%, primarily in the thick ascending limb
Reabsorption of Sodium in the Loop of Henle
In the thin descending and ascending limbs of Henle's loop, sodium and Cl- are passively reabsorbed
In the thick ascending limb sodium crosses the luminal membranes via the Na+ - H+ antiporter and by an Na+K+2Cl- cotransporter
Na+K+2Cl- cotransporter is the site of action of the loop diuretic furosemide and bumetanide
There is a strong electrochemical gradient for Na+ entry across the luminal membrane in this region
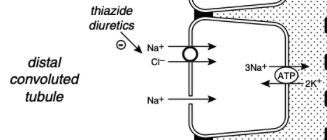
What % of the filtered load of sodium is reabsorbed in the distal convoluted tubule and the connecting segment?
5%
Sodium Reabsorption in the Distal Convoluted Tubule and Connecting Segment
In the early distal tubule (up to the connecting segment), sodium crosses the luminal membrane by means of a Na+Cl- cotransporter
This contransporter is inhibited by the thiazide diuretics
What % of the filtered load of sodium is reabsorbed in the collecting ducts?
3%
Sodium Reabsorption in the Collecting Ducts
This segment of the nephron is responsible for altering sodium reabsorption in response to dietary fluctuations
In the late distal tubule (connecting segment) and collecting ducts, sodium enters passively through Na+ channels in the luminal membranes of the principal cells
This movement of Na+ generates a lumen-negative transepithelial potential difference that facilitates Cl- reabsorption
The Na+ channel in the principal cells is blocked by the diuretic amiloride and triamterene
One of the main effects of aldosterone is to increase the number of open luminal Na+ channels in the cortical collecting ducts, altering sodium reabsorption in response to change in dietary sodium intake
Effective Circulating Volume
Relative fullness of the circulating portion of the extracellular compartment as perceived by the body
Where are low-pressure mechanoreceptors (volume receptors) located?
The cardiac atria and pulmonary vessels
Where are high-pressure baroreceptors (pressure receptors) located?
The aortic arch and carotid sinus
Renal Regulation of Sodium Balance
The juxtaglomerular apparatus responds to changes in perfusion pressure with changes in renin production and release
Kidney is the primary efferent limb of sodium control
Regulates sodium balance by excreting an amount of sodium each day equal to that ingested
The two points of control for sodium balance in the kidney are glomerular filtration and tubular reabsorption
Autoregulation maintains renal blood flow and GFR relatively constant despite fluctuations in systemic arterial pressure so the filtered load of sodium is also kept relatively constant
Effects of Changes in GFR on Sodium Balance
Slight changes in GFR have the potential to have drastic effects on sodium balance if the absolute amount of sodium reabsorbed by the tubules remains constant
If spontaneous (primary) fluctuations in GFR occur, the absolute tubular reabsorption of filtered solutes changes in a similar direction
The fraction of the filtered load that is reabsorbed remains relatively constant despite spontaneous changes in GFR
Called glomerulotubular balance
Much of the sodium in the proximal tubules is reabsorbed along with several other solutes (e.g. glucose, amino acids, phosphate, and bicarbonate)
A spontaneous increase in GFR increases the filtered load of all of these solutes and their increased concentration in the proximal tubule enhances sodium reabsorption
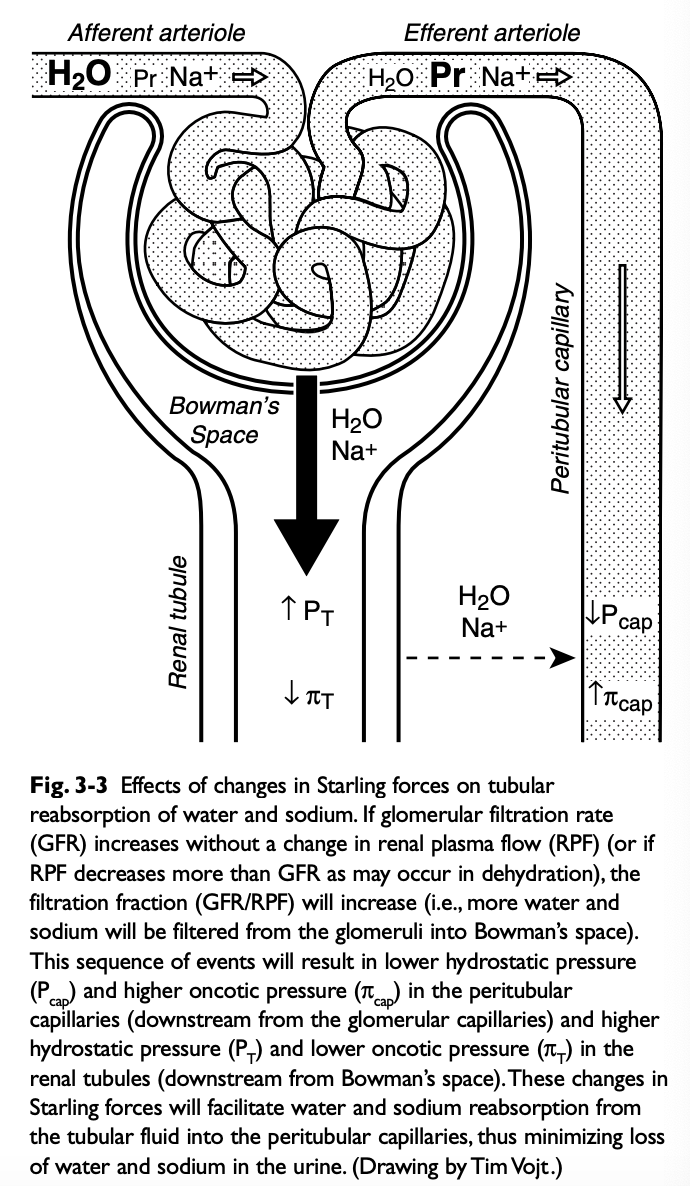
Role of Changes in Peritubular Capillary Hydrostatic and Oncotic Pressure in Glomerulotubular Balance
If GFR spontaneously increases without a change in renal plasma flow (RPF) (i.e. filtration fraction increases), the blood leaving the efferent arterioles has lower hydrostatic and higher oncotic pressure, favoring water and solute reabsorption in the proximal tubules
Contribution of Autoregulation to Glomerulotubular Balance
When renal perfusion pressure is increased, afferent arteriolar constriction prevents transmission of the increased hydrostatic pressure to the glomerular capillaries and minimizes any increase in GFR and filtered solute load
Effects of Ingestion of a Sodium Load
Autoregulation also contributes to glomerulotubular balance
When renal perfusion pressure is increased afferent arteriolar constriction prevents transmission of the increased hydrostatic pressure to the glomerular capillaries and minimizes any increase in GFR and filtered solute load
Ingestion of a sodium load causes thirst, water consumption, and expansion of ECF volume
These events lead to a compensatory (secondary) increase in GFR by increasing hydrostatic pressure and decreasing oncotic pressure in the glomerular capillaries
Increased stretching of the afferent arterioles decreases renin secretion (and ultimately angiotensin II production)
Volume expansion also causes increased atrial stretch, release of atrial natriuretic peptide, and natriuresis
What type of changes in GFR evoke glomerulotubular balance?
Glomerulotubular balance is evoked with spontaneous (primary) increases in GFR but not compensatory (secondary) increases in GFR
What is the action of aldosterone?
Changes in renal absorption of sodium in response to dietary fluctuations in sodium intake are mediated by aldosterone
Aldosterone increases sodium reabsorption by increasing the number and activity of open sodium channels in the luminal membranes of the principal cells in the collecting ducts
Where is aldosterone produced?
The zona glomerulosa of the adrenal cortex
What stimulates production and release of aldosterone?
Angiotensin II, hyperkalemia, and ACTH
What inhibits release of aldosterone?
Dopamine and atrial natriuretic peptide
Effects of Increased Sodium Intake on Peritubular Capillary Factors (Starling Forces)
Increased sodium intake leads to expansion of the ECF volume and compensatory increases in GFR and renal plasma flow (i.e. filtration fraction remains unchanged)
This increases hydrostatic pressure and decreases oncotic pressure in the peritubular capillaries, reducing sodium and water reabsorption in the proximal tubules
Effects of Decreased Sodium Intake on Peritubular Capillary Factors (Starling Forces)
Decreased sodium intake leads to volume contraction
RPF decreases more than GFR (filtration fraction increases)
Results in decreased hydrostatic pressure and increased oncotic pressure in the peritubular capillaries and enhanced proximal tubular reabsorption
Effects of Catecholamines on the Efferent and Afferent Arterioles
Catecholamine-induced vasoconstriction usually affects the efferent more than the afferent arterioles
The resultant increase in filtration fraction alters peritubular capillary hemodynamics to favor water and sodium reabsorption (decreased hydrostatic pressure and increased oncotic pressure)
Effects of Catecholamines on Sodium Reabsorption
Catecholamines directly stimulate proximal tubular sodium reabsorption through an a1-adrenergic effect and stimulate renin release from the granular cells of the juxtaglomerular apparatus through a B1-adrenergic effect
The angiotensin II ultimately produced also stimulates proximal tubular sodium reabsorption
The direct effects of catecholamines on proximal tubular sodium reabsorption offset the tendency of the increase in systemic arterial pressure to cause natriuresis
What leads to production of angiotensin II?
Decreased perfusion pressure in the afferent arterioles increases renin release from the granular cells of the juxtaglomerular apparatus and initiates the events that result in production of angiotensin II
Effects of Angiotensin II
Angiotensin II-induced vasoconstriction causes efferent more than afferent arteriolar constriction
Results in an increase in filtration fraction and changes in peritubular capillary Starling forces (decreased hydrostatic pressure and increased oncotic pressure) that facilitate proximal tubular reabsorption of sodium and water
Angiotensin II also directly stimulates the Na+H+ antiporter in the proximal tubules, which facilitates sodium reabsorption and stimulates secretion of aldosterone from the adrenal gland
Where is atrial natriuretic peptide produced?
ANP is synthesized and stored in atrial myocytes
What stimulates the release of ANP?
Released in response to atrial distension caused by volume expansion
Effects of ANP
Effects of ANP facilitate renal excretion of sodium
Causes dilation of the afferent arterioles and constriction of the efferent arterioles, leading to a primary increase in GFR
Relaxes mesangial cells, resulting in an increase in the glomerular surface area available for filtration
Inhibits sodium reabsorption in the cortical and inner medullary collecting ducts and inhibits renin secretion, decreasing the production of angiotensin II
Inhibits aldosterone secretion by adrenal zona glomerulosa
Pressure Natriuresis
Renal sodium excretion and water excretion are markedly increased when renal arterial pressure increases even slightly without a change in GFR
Mechanism appears entirely intrarenal
Stimuli for Release of Aldosterone
Angiotensin II
Hyperkalemia
Adrenocorticotropic hormone
Inhibitors of Release of Aldosterone
Dopamine
ANP
Major Effects of Aldosterone
Increased number and activity of luminal Na+ channels and basolateral Na+,K+ ATPase in principal cells of cortical collecting ducts
Stimuli for Release of Angiotensin II
Decreased renal perfusion pressure
Inhibitors of Angiotensin II Release
Increased renal perfusion pressure
Major Effects of Angiotensin II
Systemic vasoconstriction
Glomerular arteriolar vasoconstriction (efferent >afferent)
Stimulates proximal Na+ reabsorption
Stimulates aldosterone secretion
Stimuli for Release of Atrial Natriuretic Peptide (ANP)
Increased atrial stretch
Inhibitors of Release of Atrial Natriuretic Peptide (ANP)
Decreased atrial stretch
Major Effects of Atrial Natriuretic Peptide (ANP)
Inhibits Na+ reabsorption in parts of collection duct
Directly increases glomerular filtration rate
Stimuli for Release of Catecholamines
Decreased effective circulating volume
Inhibitors of Release of Catecholamines
Increased effective circulating volume
Major Effects of Catecholamines
Vasoconstriction
Glomerular arteriolar vasoconstriction (efferent > afferent)
Increase proximal tubular Na+ reabsorption
(a1 effect)
Stimulate renin release (B1 effect)