PMB 4412 Exam 2
1/135
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
136 Terms
two types of energy converting organelles
chloroplasts
plastids, found only in plants and algae
mitochondria
both are separated from the cytosol by a double membrane
characteristics of mitochondria and chloroplasts
semi-autonomous
divide by fission
contain circular chromosomal DNA, located in nucleoids within the stroma and matrix
contain ribosomes, tRNAs
depend on import of nuclear encoded proteins for many functions
generate transmembrane hydrogen gradient and use gradient to make ATP
overall formula for photosynthesis
6 CO2 + 6 H2O → C6H12O6 + 6O2
what happens during the light reactions of photosynthesis?
energy from light is used to phosphorylate ADP (produce ATP) and to reduce NADP+ to NADPH
in chloroplasts, the light reactions drive _______ of the thylakoid lumen
acidification
where is ATP generated in chloroplasts? where is ATP generated in mitochondria?
in chloroplasts = stroma
in mitochondria = matrix
A __________ can be formed when membranes allow selective permeation
diffusion potential
exists until chemical equilibrium is reached → then diffusion potential equals 0
diffusion is passive transport
what is the plant cell membrane potential inside relative to outside?
negative inside, positive outside
H+ ATPase pumps protons out
how does inhibiting ATP synthesis affect membrane potential?
membrane is depolarized
H+ ATPase pumps out protons, makes the inside of the membrane negative and the outside positive
turning off ATP synthesis mean means no more ATP hydrolysis, H+ ATPase becomes inactive
inside of the membrane becomes more positive
what forms of transport across membranes are passive?
diffusion
channels
uniporter: binds substrate on one side, changes conformation, releases substrate on other side
what forms of transport across membranes are active?
pump
symporter
antiporters
use energy to move something against its electrochemical gradient
molecular structure of H+ ATPase
both N and C terminus in cytoplasm
several transmembrane domains
transmembrane domains have occasional charged amino acids embedded in the membrane
these charged amino acids have special functions
regulatory domains within the cytoplasm
have bindings sites for Mg2+
phosphorylation domain
structure of H+ ATPase in vacuole membrane
domains in cytoplasm spin
channel allows proton flow from the cytoplasm to the lumen of the vacuole
hydrolyze ATP to move protons
process is reversible, based on stoich of protons to ATP
difference between H+ ATPase and ATP synthase
H+ ATPase hydrolyzes ATP to pump protons against their gradient -→ out of the cell or into the vacuole
ATP synthase makes ATP using the power of protons moving down their gradient
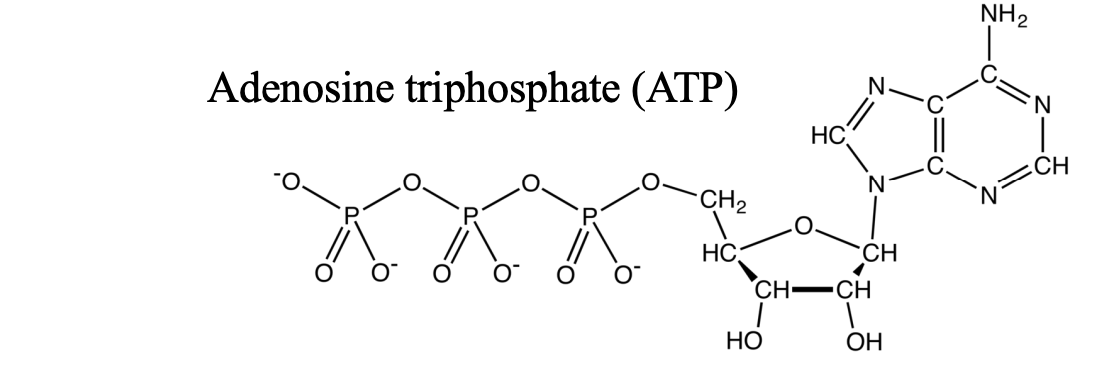
structure of ATP
five carbon ribose sugar
adenine nitrogenous base
3 phosphates
3 phosphate linkage is unstable because negative charges are next to each other → hydrolysis
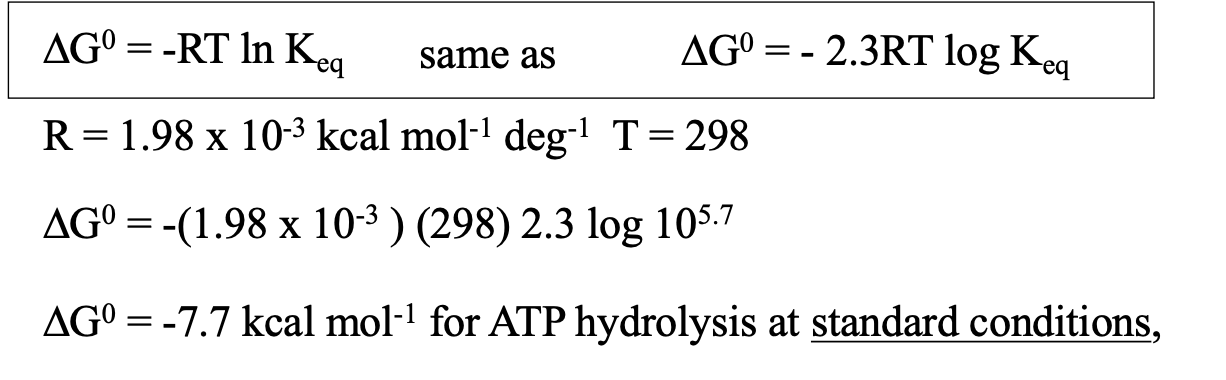
what are standard conditions?
25 degrees Celsius
1 M products and reactants
not cellular conditions
what is Keq?
concentration of products over concentration of reactants AT EQUILIBRIUM
when Keq is big = more products than reactants at equilibrium
when Keq is small = more reactants than products at equilibrium
ATP hydrolysis under standard conditions
ATP → ADP + Pi
Keq is large = ATP is unstable = wants to lose P
standard conditions are not cellular conditions
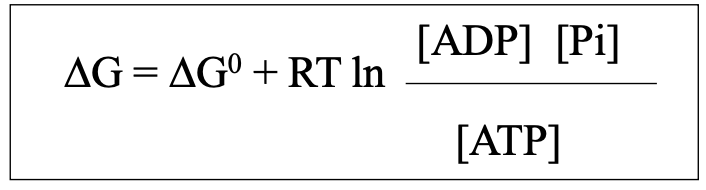
energy of ATP hydrolysis in the cell (no electrical component)
dependent on concentration of ATP and hydrolysis products
how do we consider just the electrical component of membrane potential?
energy required to transport one mole of charge against a membrane potential

how do we consider just the concentration component of membrane potential?
energy required to transport one mole of solute against a concentration gradient

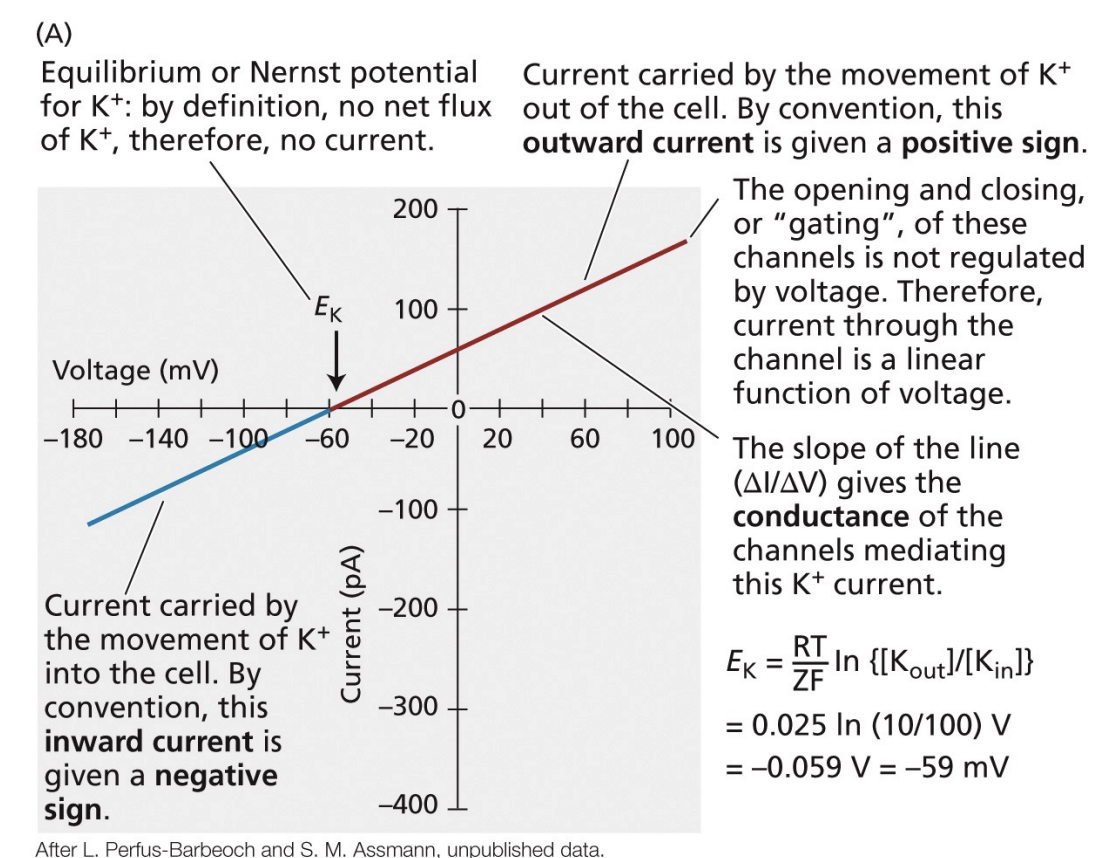
what is equilibrium potential?
membrane potential at which the given ion concentrations are at equilibrium
how do we determine the equilibrium potential for a given ion?
Nernst potential
Walther Hermann Nernst
takes into account electrical and concentration components
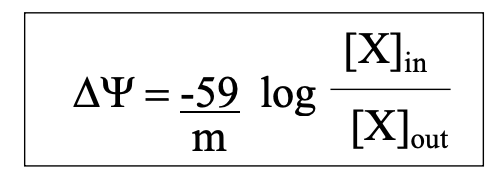
graphing of equilibrium potentials
at zero, no net flux of the ion
slope of the line gives the conductance of the channels mediating the current
for cations
movement out of the cell = outward current = positive sign
movement into the cell = inward current = negative sign
for anions
movement out of the cell = negative sign
movement into the cell = positive sign
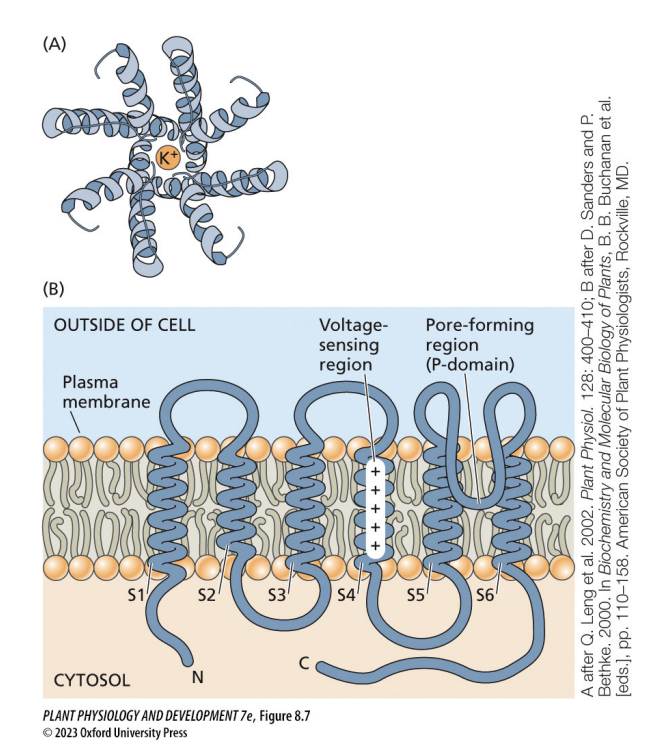
how do voltage-gated channels sense changes in voltage?
charged amino acids within the transmembrane spans sense changes in voltage of the membrane
channels change conformation in response to voltage changes
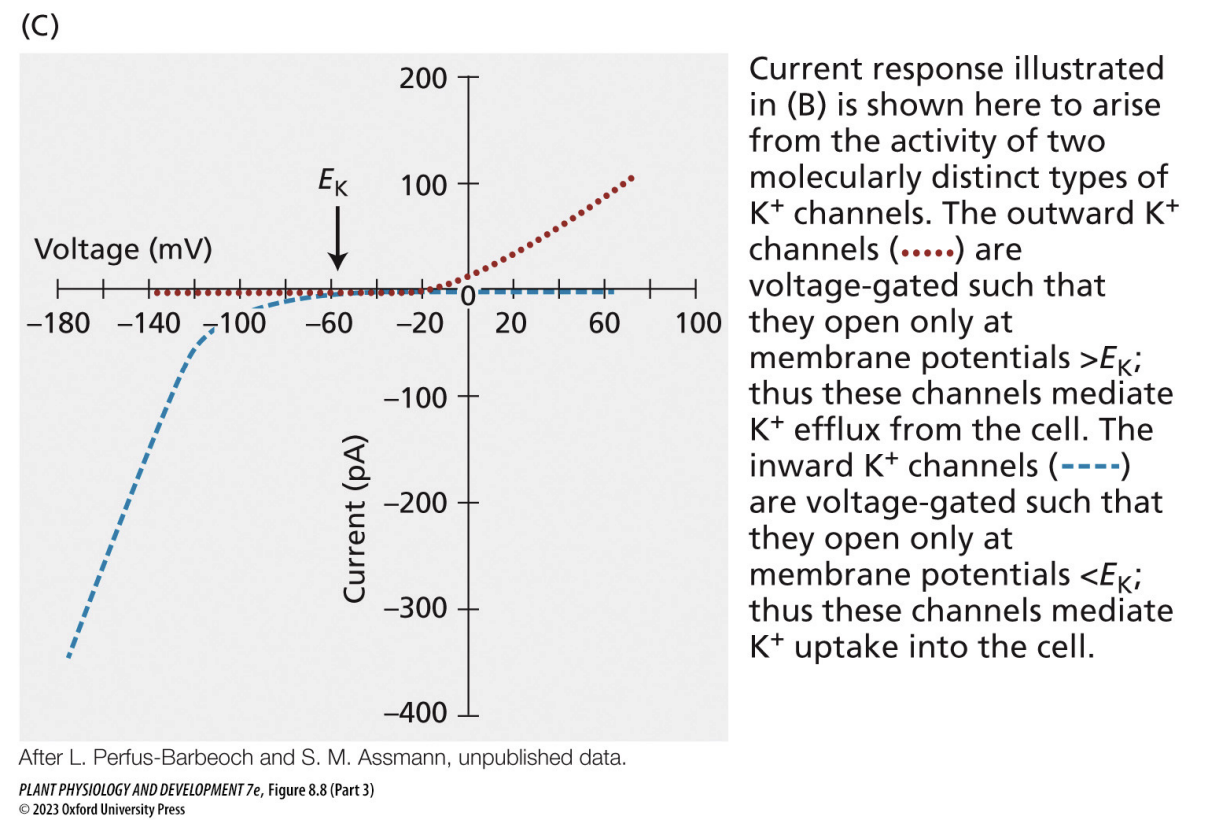
how can we use equilibrium potential graphs to determine what kind of channels ions are moving through?
outward channels open when membrane potential exceeds equilibrium potential for potassium → potassium moves out
inward channels open when membrane potential is below equilibrium potential for potassium → potassium moves in
“goal” = get the membrane potential as close to the ion equilibrium potential as possible
what is channel gating?
opening and closing of ion or solute channels
involves protein conformation change
once open, ions or solutes are conducted at very fast rates
what is voltage dependence?
regulation of the channel by membrane potential
open/close in response to voltage
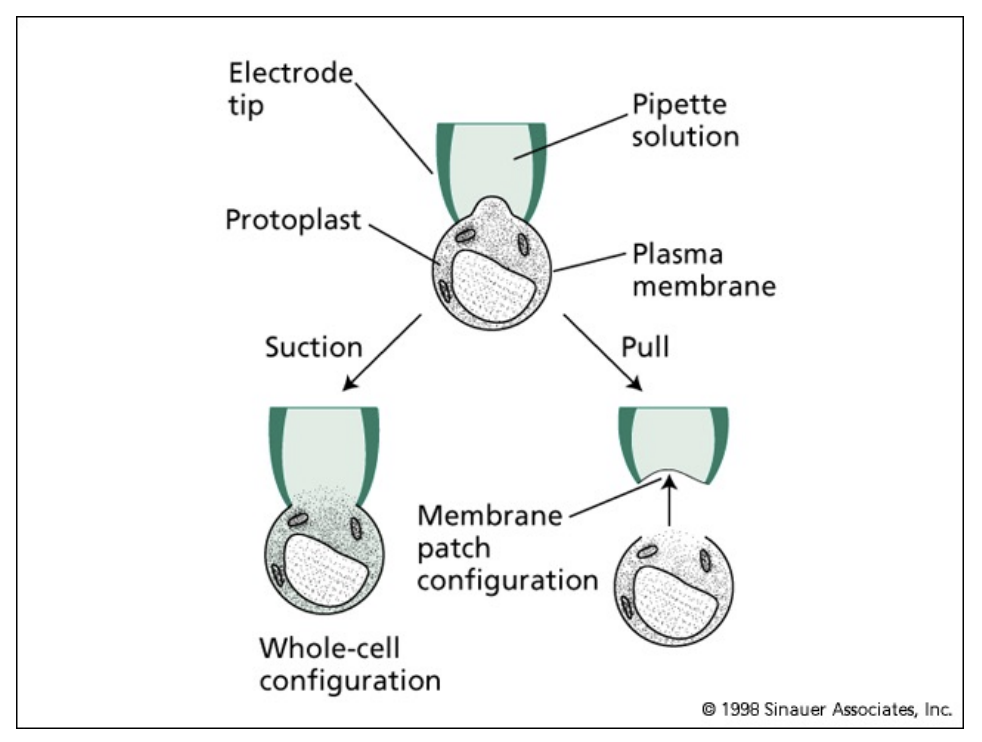
how can we measure ion channels?
patch clamping
discovered by Erwin Neher and Bert Sakmann
electrophysiology methods
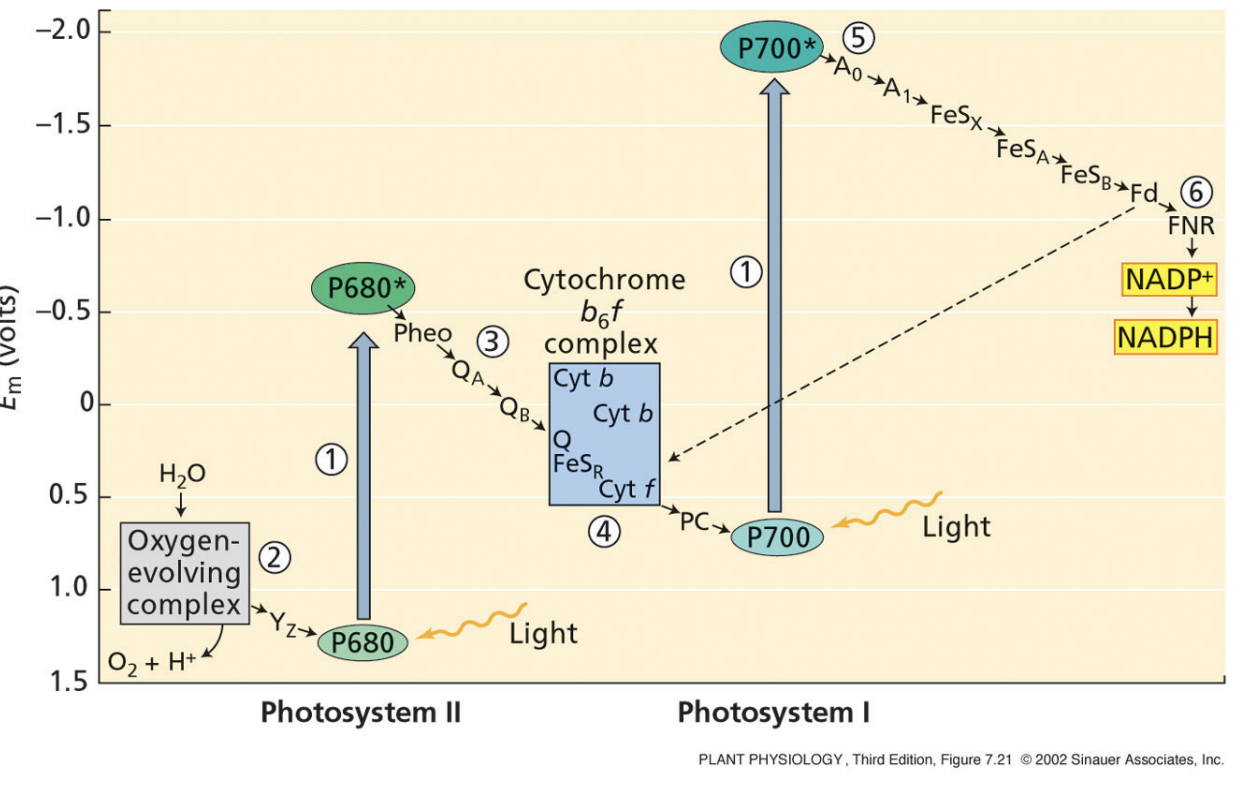
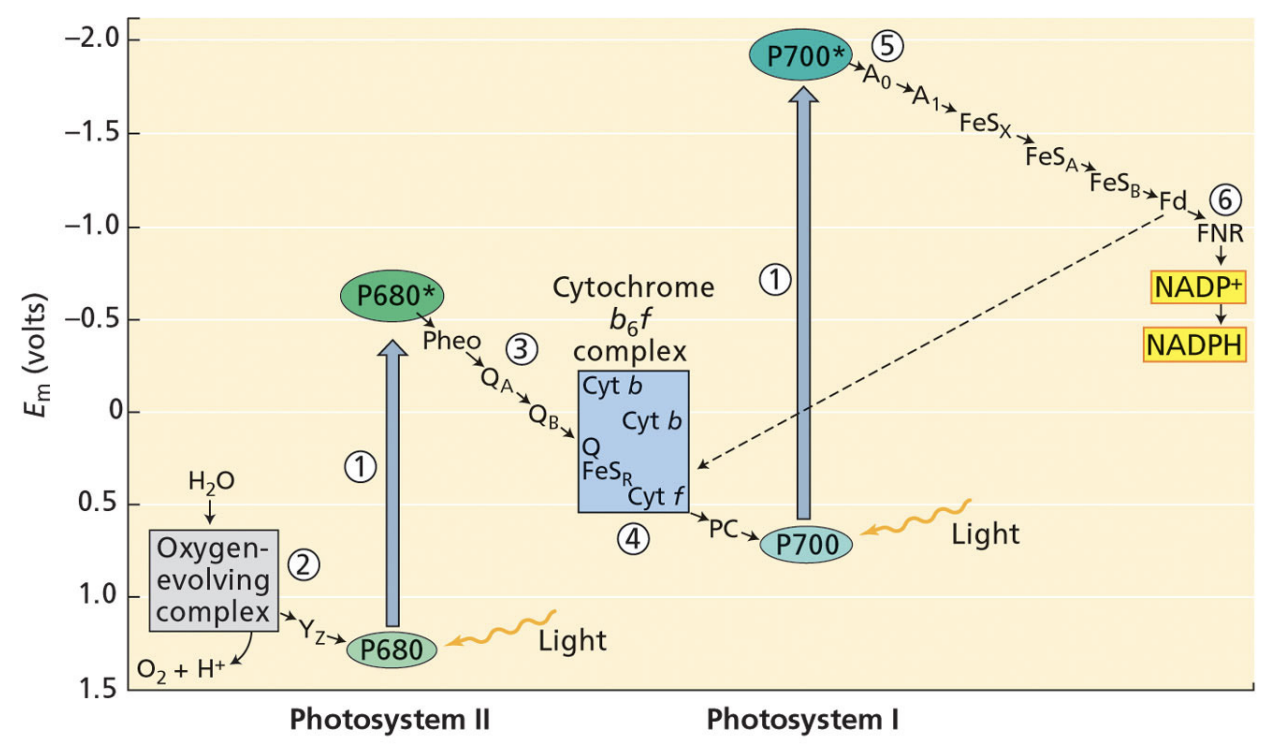
what is the first key reaction of photosynthesis?
splitting water
2 H2O → 4 e + 4 H+ + O2
done by O2-evolving complex that contains Mn
O2 is a waste product
negative redox potential
what is redox potential mean?
negative redox potential = more willing to give up electrons
positive redox potential = more willing to take up electrons
electrons flow spontaneously from negative redox potentials to positive redox potentials
in the light reactions of photosynthesis, hydrogen is stockpiled into the __________, flows out the ATP synthase channel into the ________, and makes ATP in the __________
thylakoid lumen
stroma
stroma
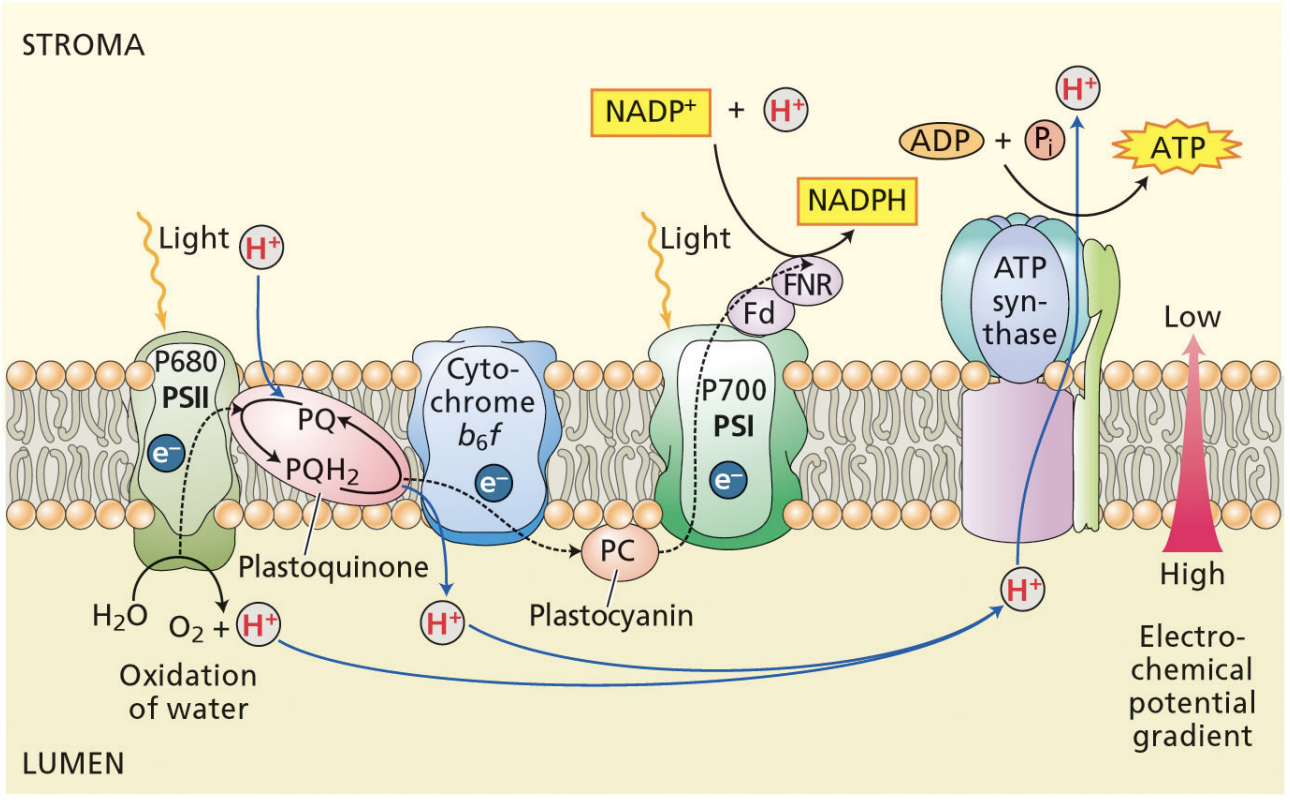
PSII is located primarily in the ________________
stacked grana
PSII
light oxidizes P680
P680 is reduced by the electrons from splitting H2O, protons from water are left in lumen
Reduced P680 passes electrons to a pheophytin (chlorophyll)
Pheophytin passes electrons to plastoquinones QA and QB
QB is reduced by 2 electrons and 2 protons → protons are taken from the stroma
Cytochrome b6f takes electrons from QB, the protons from QB are pumped into the lumen
4 H+ pumped in for each 2 electrons that go through cyt b6f
cytochrome b6f passes electrons to plastocyanin (final electron acceptor of PSII
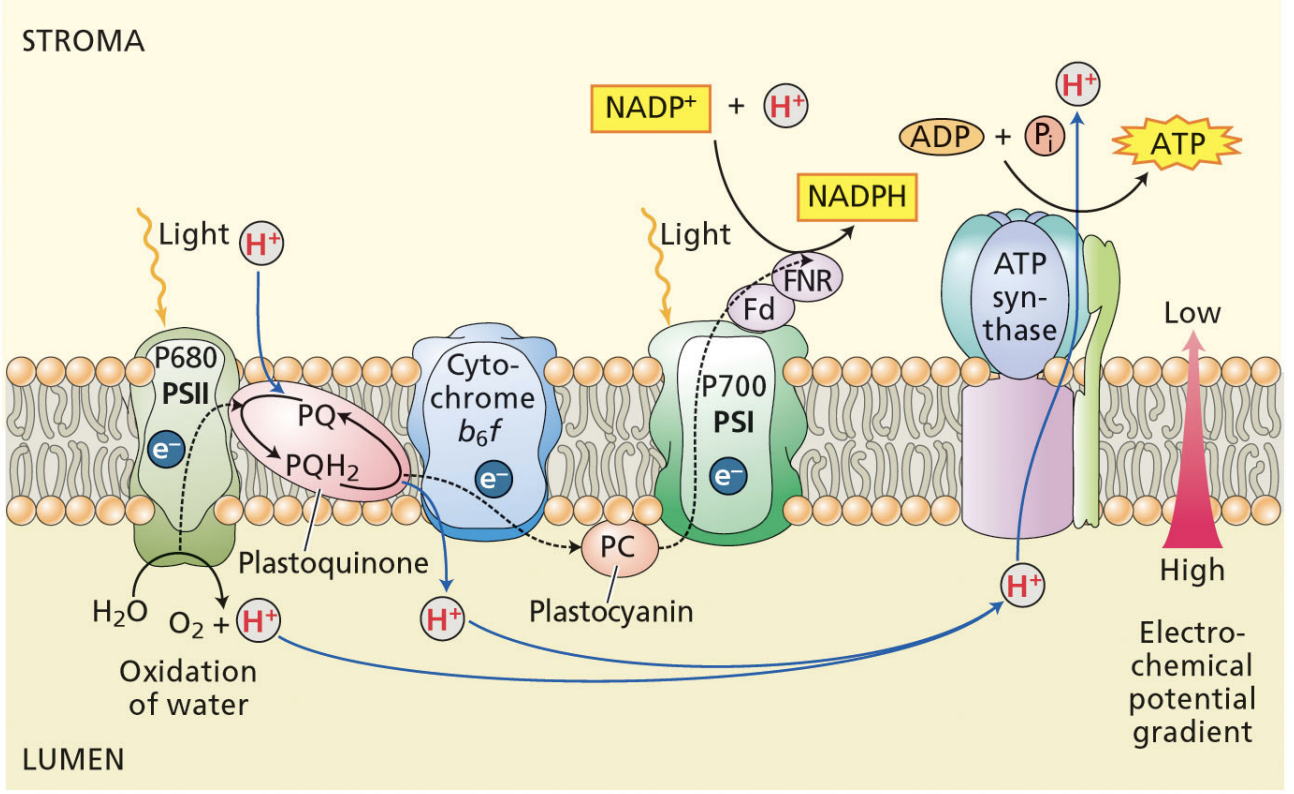
4 ways protons are moved into thylakoid lumen during PSII
splitting of water by oxygen-evolving complex → protons in the lumen
plastoquinones take up protons from the stroma → protons removed from stroma
protons are pumped into the lumen when plastoquinones pass electrons to cyt b6f → protons in the lumen
protons are used to reduce NADP+ in the stroma → protons removed from the stroma
final electron acceptor of PSII
plastocyanin
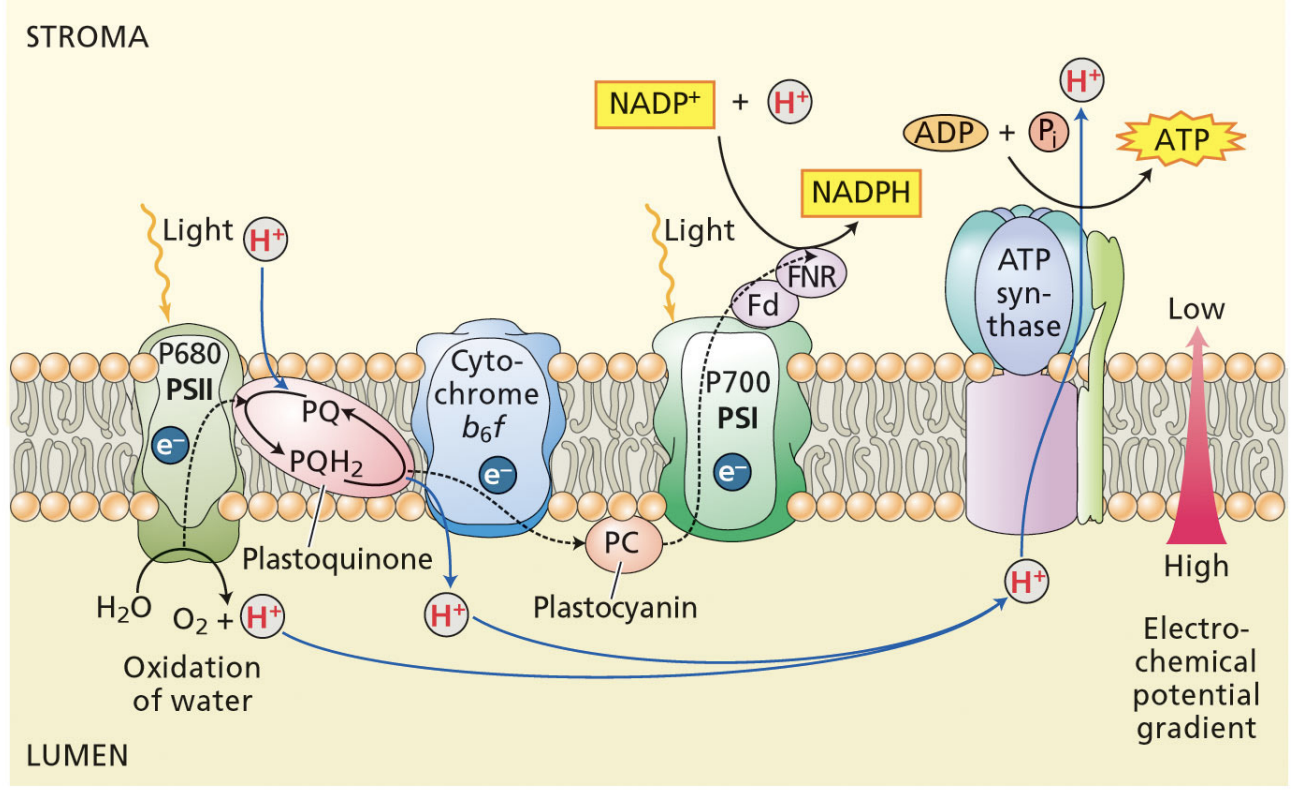
initial electron donor of PSII
H2O
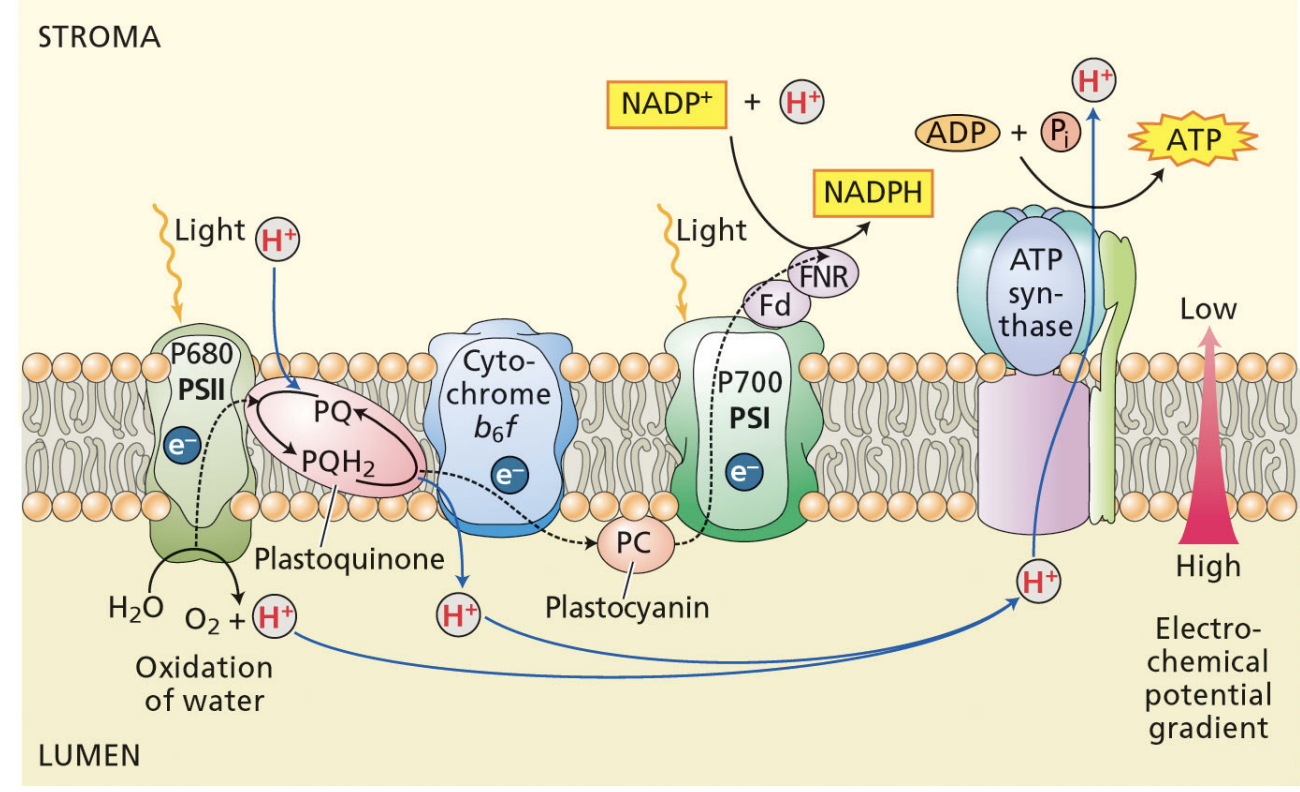
initial electron donor of PSI
plastocyanin
where is PSI and ATP synthase?
stromal thylakoids
unstacked regions
PSI
light oxidizes P700
plastocyanin reduces P700
P700 reduces A0 quinone
A0 quinones transfers electrons through a series of FeS proteins
FeSB reduces soluble ferredoxin
reduced ferredoxin transfers electrons to FNR
FNR reduces NADP+ to NADPH → removes protons from the stroma
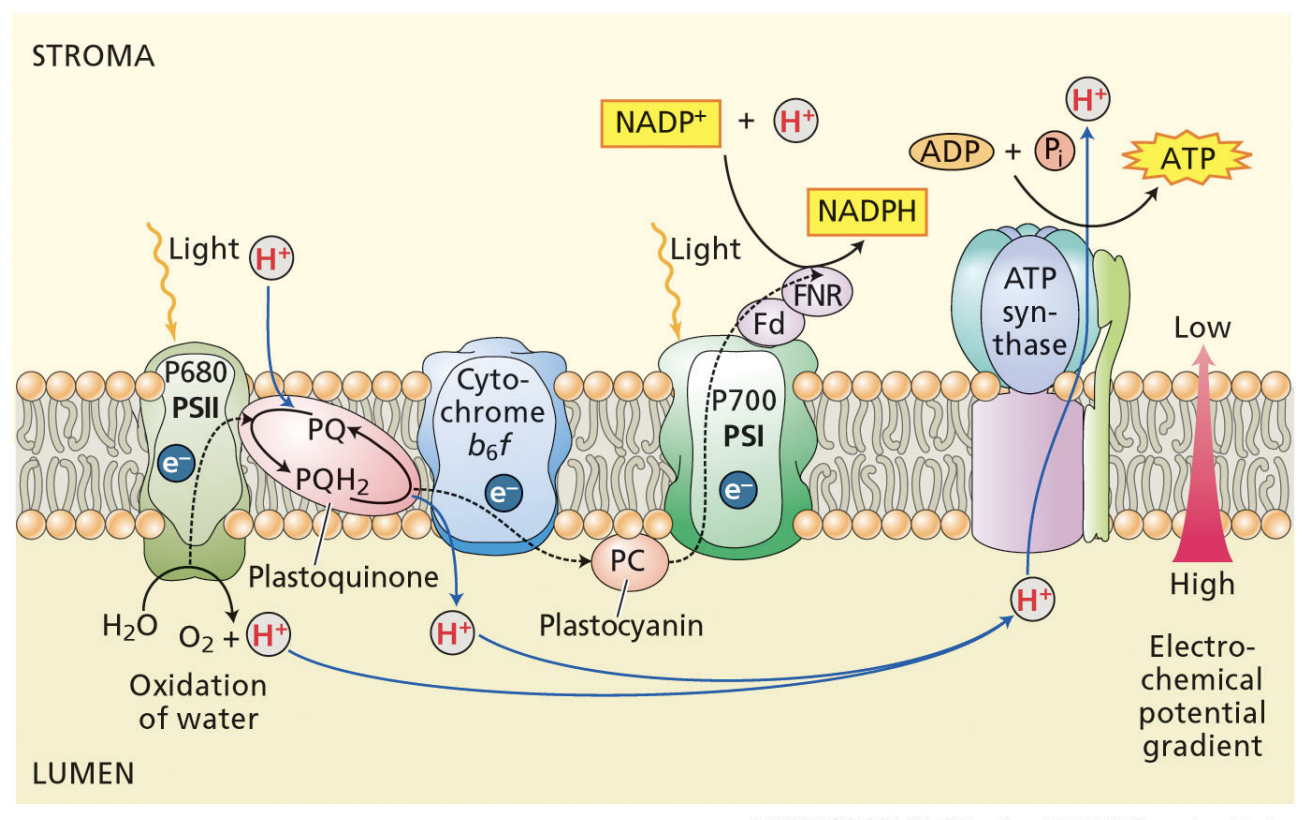
what are the products of the light reactions?
O2
NADPH → used in the Calvin cycle to reduce CO2
ATP
ultimate electron donor for the light reactions?
H2O
ultimate electron acceptor for the light reactions?
NADP+
how does the ATP synthase use the proton gradient to make ATP?
proton concentration is much higher in the thylakoid lumen than in the stroma
protons move down their gradient from thylakoid lumen to the stroma through a channel in the ATP synthase
proton motive force powers the phosphorylation of ADP into ATP in the stroma
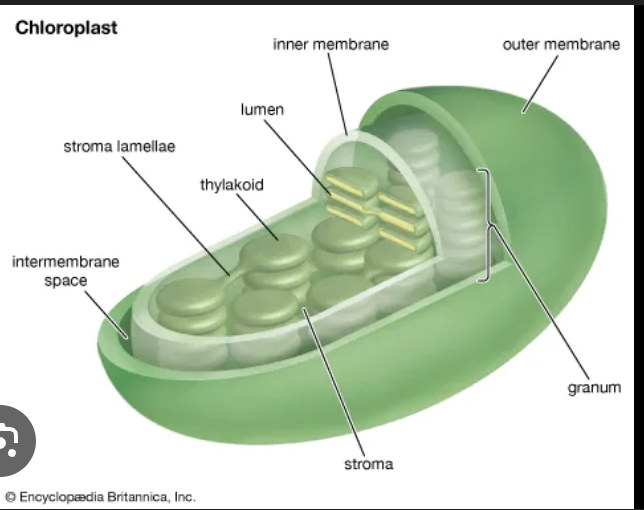
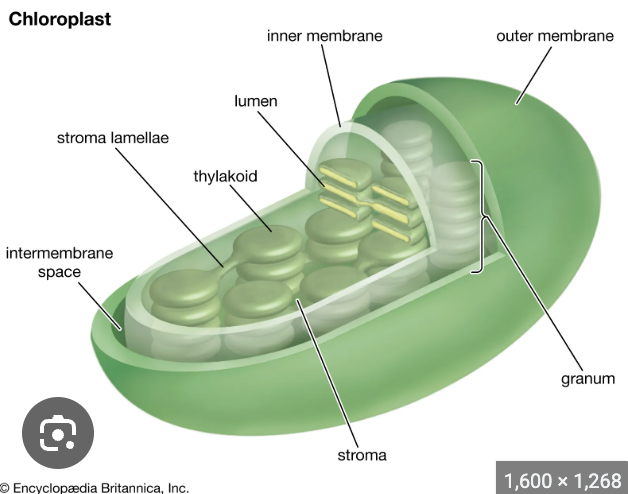
important features of thylakoid structure
continuous thylakoid lumen
extensive contact and continuity between stromal and thylakoid membranes
what ion is required for the splitting of H2O in PSII?
need 4 Mn ions
what does FNR stand for?
ferredoxin NADP+ reductase
FNR reduces NADP+ to NADPH → removes proton from stroma and gives NADP+ electrons
what is cyclic electron transport?
chloroplast can redirect electrons from ferredoxin to cyt b6f to make more ATP rather than making more NADPH
function of the light reactions of photosynthesis
generate ATP and NADPH
function of the carbon reactions of photosynthesis
fix CO2 and regenerate ribulose-1,5-bisphosphate
requires NADPH and ATP
light reactions occur on ______________
thylakoid membrane
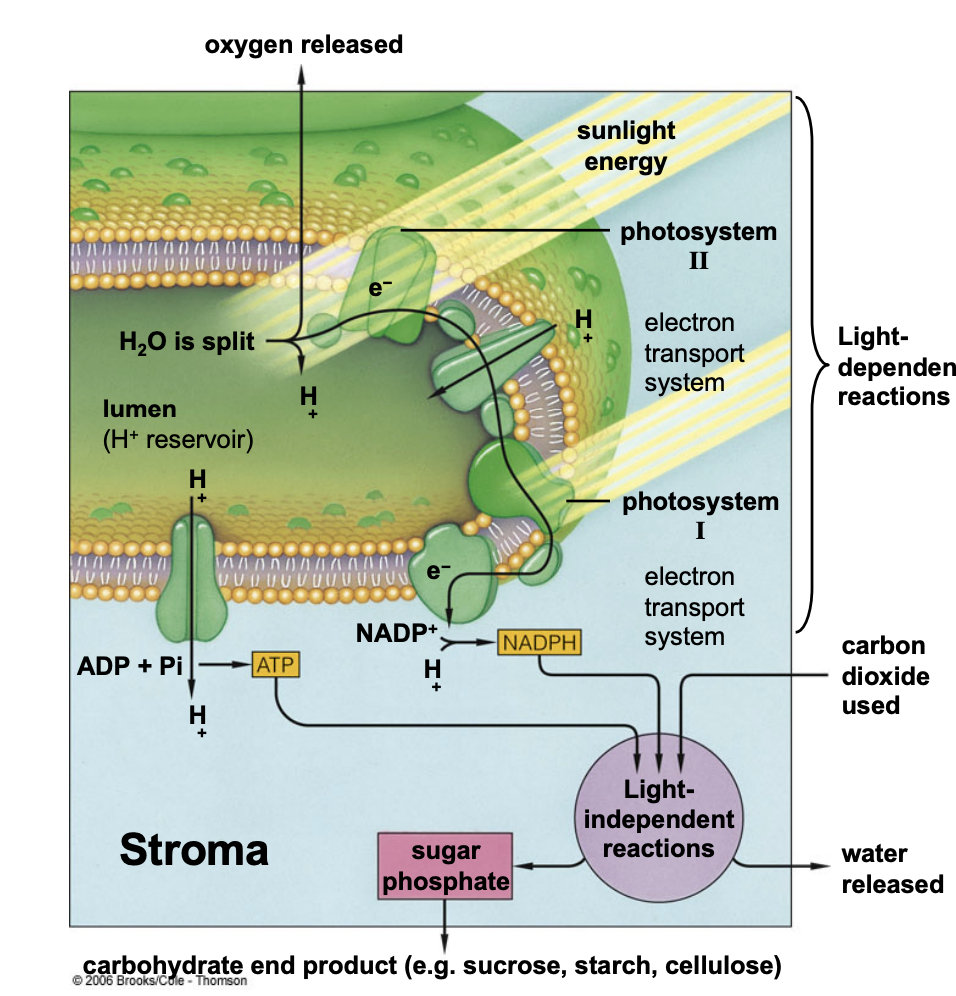
carbon reactions occur in the _________
stroma

NADP+ is reduced to NADPH during __________ in the ___________
PSI
stroma
what is FNR?
protein in the stroma
exists as either a soluble monomer (inactive) or a thylakoid bound dimer (active)
NOT A TRANSMEMBRANE PROTEIN
contains a FAD cofactor → uses reducing power of two ferredoxin to reduce NADP+ → NADPH
how did Melvin Calvin discover the carbon reactions?
used radioactive carbon dioxide C14
give to algae
rapid sampling
2D chromatograph to identify metabolic intermediates
what is the first stable product of carboxylation in the carbon cycle?
3PGA
ribulose-1,5-bisphosphate + CO2 → Rubisco → 3PGA
where do the carbon reactions (Calvin cycle) of photosynthesis happen?
stroma
where is Rubisco found?
chloroplast stroma
what are the substrates and products of Rubisco?
ribulose-1,5-bisphosphate + CO2 → 3PGA
what are the three stages of the Calvin cycle?
Carboxylation of ribulose-1,5-bisphosphate with CO2 to 3PGA
reduction using ATP and NADPH to G3P and DHAP → can be used to regenerate ribulose-1,5-bisphosphate or to make sucrose, starch
regeneration of ribulose-1,5-bisphosphate using ATP
what are the triose phosphates?
DHAP and G3P
what are the two uses of G3P?
used to make sucrose and starch
combined with DHAP to regenerate ribulose-1,5-bisphosphate
where is starch synthesized?
stroma
where is sucrose synthesized?
cytoplasm
DHAP and G3P transported to the cytoplasm in exchange for phosphate
then made into sucrose
what does Rubisco mean?
ribulose-1,5-bisphosphate carboxylase/oxygenase
what activates Rubisco?
increasing stromal pH
increasing concentration of Mg2+ in the stroma
four light regulated enzymes in the Calvin cycle are activated by the ferredoxin-thioredoxin pathway → ensures that Calvin cycle happens when light reactions are happening
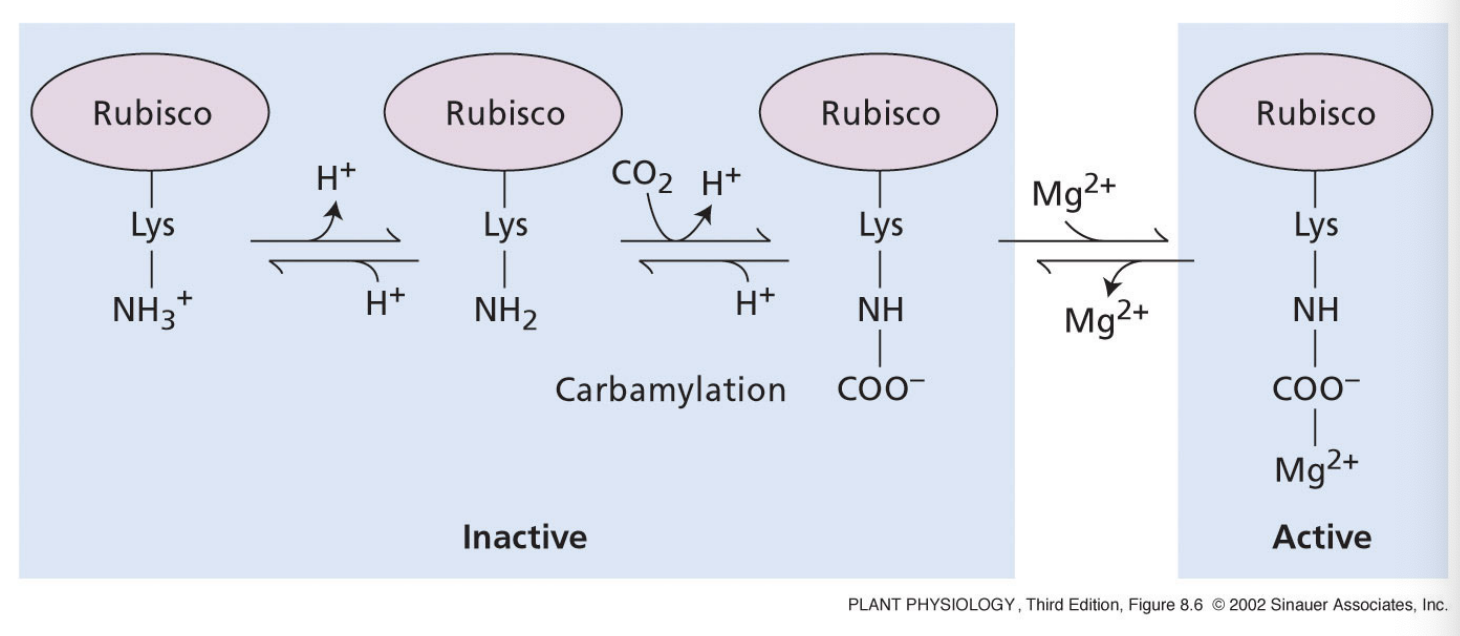
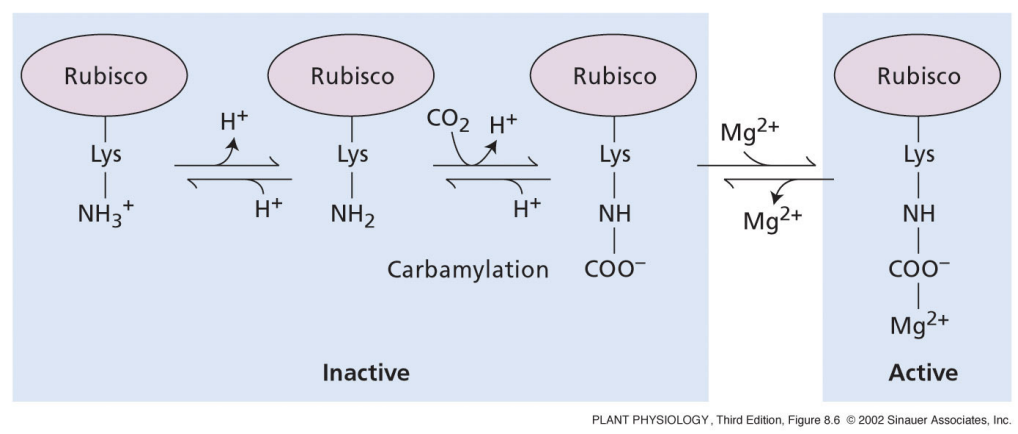
why does increasing Mg2+ concentration activate Rubisco?
light reactions create a membrane potential across the thylakoid
membrane potential is negative on stromal side
Mg2+ is driven from thylakoid lumen to negative stroma
what are the substrates and products of Rubisco when it is acting as an oxygenase?
ribulose-1,5-bisphosphate + O2 → Rubisco → Phosphoglycolate + 3PGA
PHOTORESPIRATION
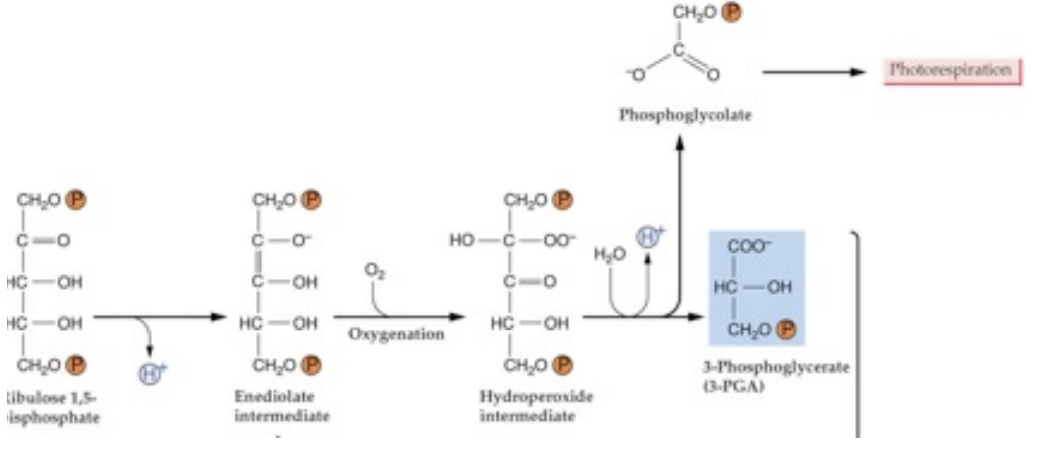
when is photorespiration more dominant?
high temperatures and dry conditions
more O2 than CO2 in solution at high temperatures
stomatal pores close in drought to conserve water → CO2 in leaves is low
why is photorespiration wasteful?
because we need to convert glycolate to a useful form and carbon is lost in the process →turns glycolate into 3PGA
requires 2.5 ATP and NADPH per glycolate because a CO2 is lost in the process and 1 ATP is required to make 3PGA
what is the primary carboxylation for C3 photosynthesis?
ribulose-1,5-bisphosphate + CO2 → Rubisco → 3PGA
happens in the stroma
what is the goal of C4?
avoid photorespiration by separating carboxylation from Rubisco
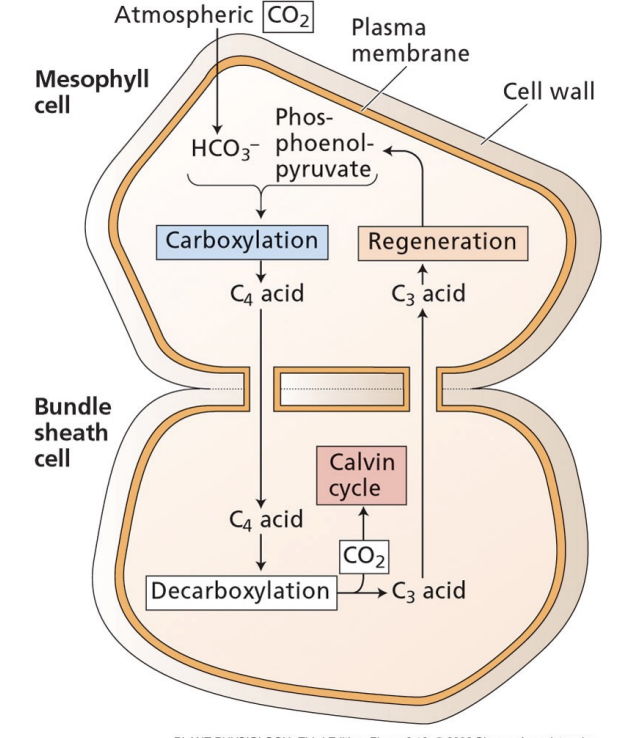
what is the primary carboxylation for C4 photosynthesis?
HCO3- + PEP → PEP carboxylase → oxaloacetate
occurs in mesophyll cells
oxaloacetate converted to C4 acid and is exported to bundle sheath cells
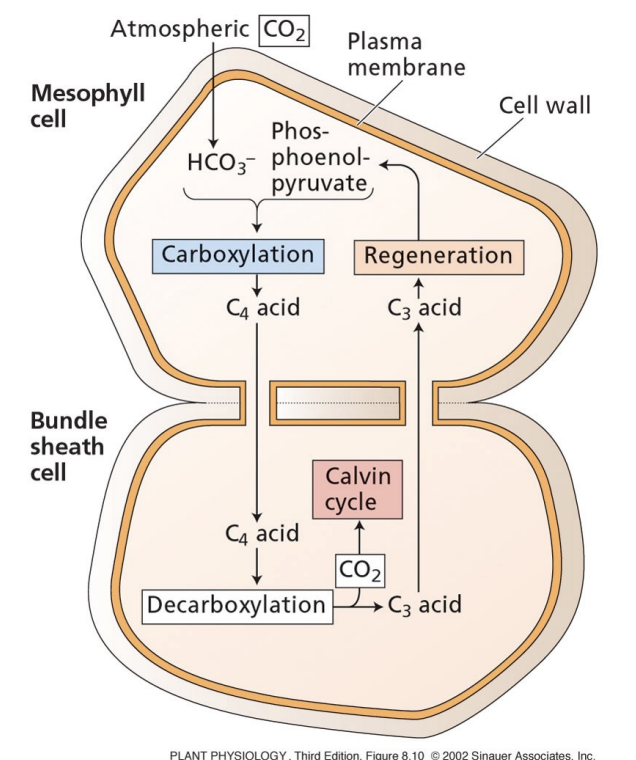
where does the Calvin cycle happen in C4 photosynthesis?
oxaloacetate made in the mesophyll cells are exported to bundle sheath cells
C4 acid is decarboxylated
decarboxylated product is combined with CO2 and carbon cycle starts
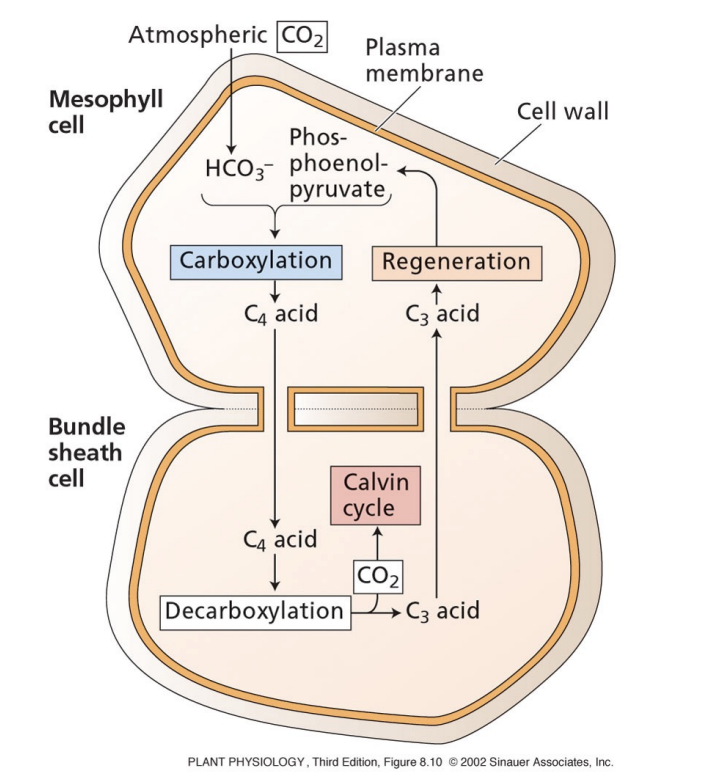
what is the cost of C4 photosynthesis?
you have to accumulate CO2 in bundle sheath cells → 2 ATP per CO2
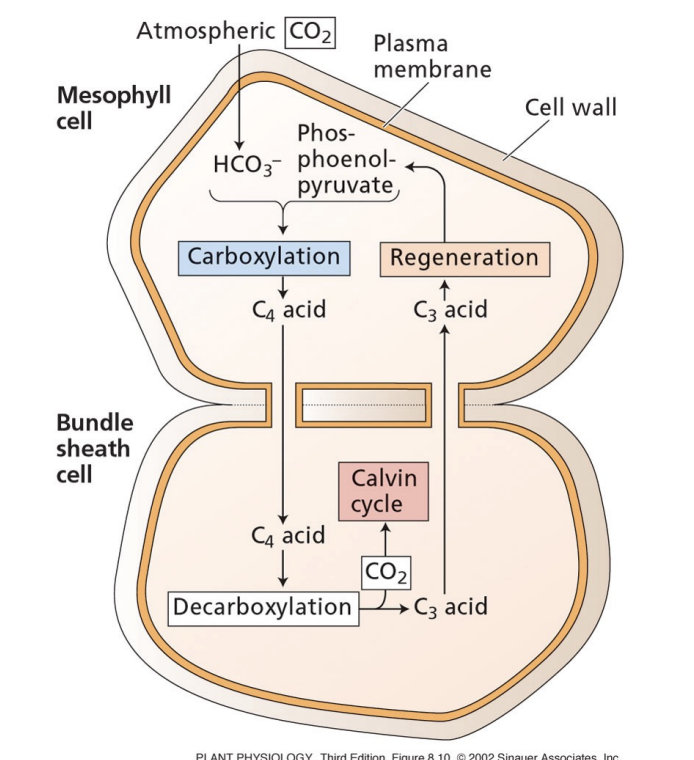
why do bundle sheath chloroplasts have little PSII?
PSII begins with splitting of H2O
produces O2
high levels of O2 = photorespiration = defeats the purpose of C4 photosynthesis
how is PEP carboxylase regulated?
light regulated
in the light → phosphorylated by PEP carboxylase kinase→ activated
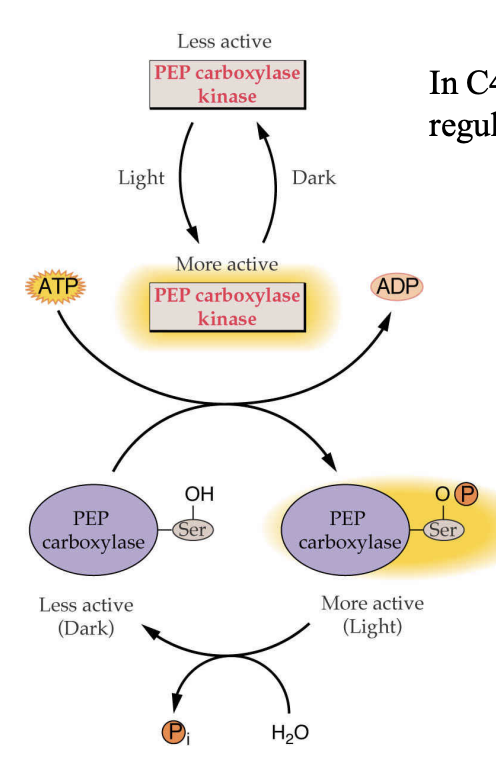
how is pyruvate dikinase regulated?
pyruvate dikinase
used to regenerate PEP
regulated by light through phosphorylation
phosphorylated in the dark = inactivated
ADP is the phosphate donor = generates AMP
when photosynthesis is low, high levels of ADP = pyruvate dikinase inactivated in the dark
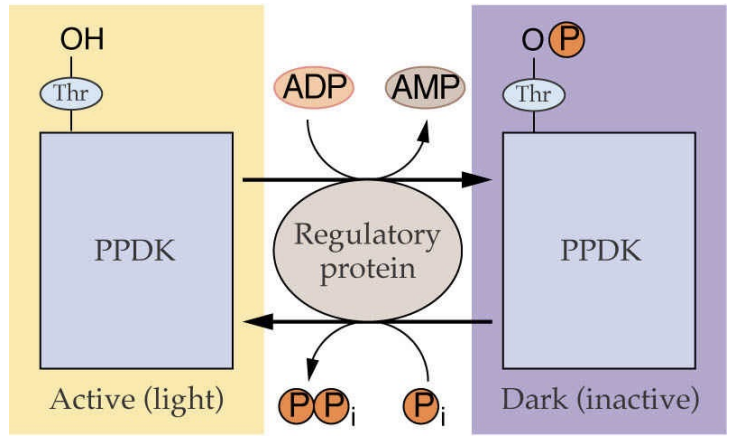
what is Kranz anatomy in plants?
in C4 plants
enlarged bundle sheath cells surround vascular bundles → high concentration of CO2 in the bundle sheath cells → very little PSII, very little stacking
mesophyll cells surround the enlarged bundle sheath cells
in C3 plants, mesophyll cells are adjacent to the vascular bundles
function of CAM
separates Calvin cycle and carboxylation by time
prevent water loss in arid environments
open stomata during cool night and close stomata during hot day
night in CAM plants
stomata are open
PEP is made from broken down starch, exported from chloroplast to cytoplasm
CO2 comes in → PEP + HCO3- decarboxylated by PEP carboxylase→ C4 acid stored in vacuole
pH is low
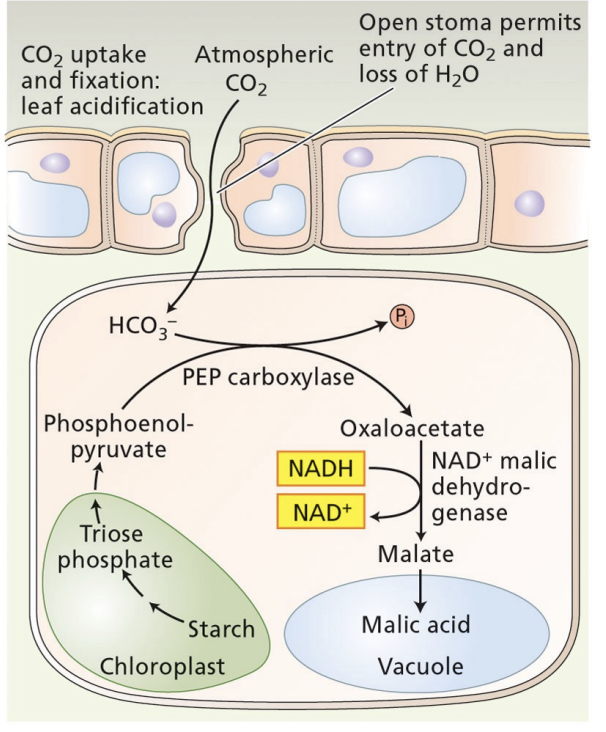
day in CAM plants
stomata are closed
C4 acid is exported from vacuole to chloroplast
C4 acid is decarboxylated and Calvin cycle happens
pH is high
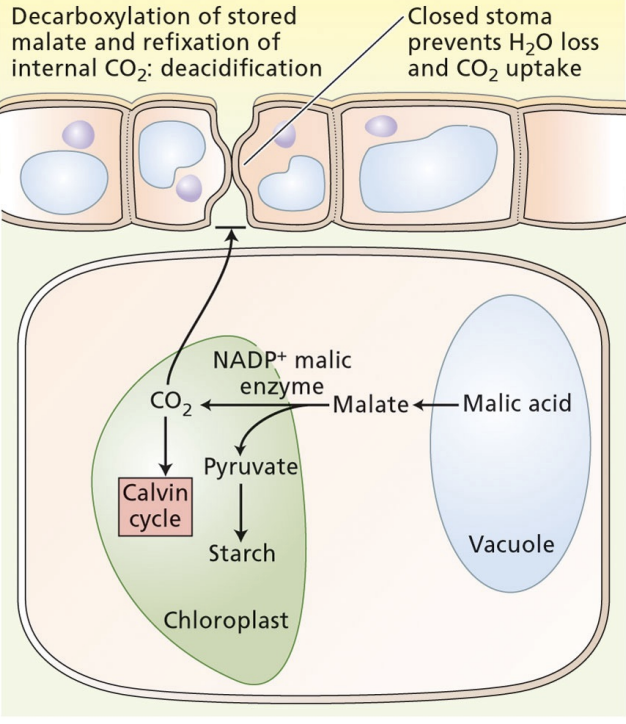
cost of CAM photosynthesis
ATP is required to transport C4 acid in and out of the vacuole
ATP is required to regenerate PEP
CO2 uptake is limited by vacuole storage space
why is excess light dangerous to plants?
excess light causes the creation of reactive oxygen radicals
over-excitation of PSII can transfer excess energy to O2 to create a free radical
can cause cellular damage
how do carotenoids protect against excess light?
carotenoids are in LHCII
carotenoids quench the excited state of chlorophyll and dissipate energy as heat
violaxanthin is converted to zeaxanthin, releases energy as heat
protects against formation of free radicals from light damage
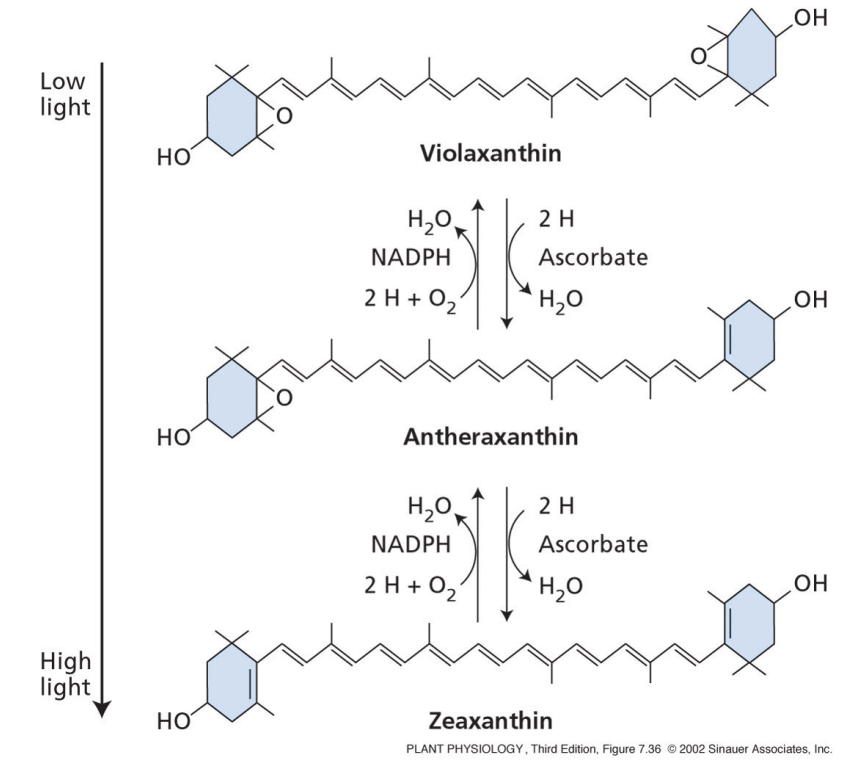
why is carotenoid protection from excess light important during water stress conditions?
in water stress, stomata is closed and CO2 photosynthesis is limited
good way to dissipate heat
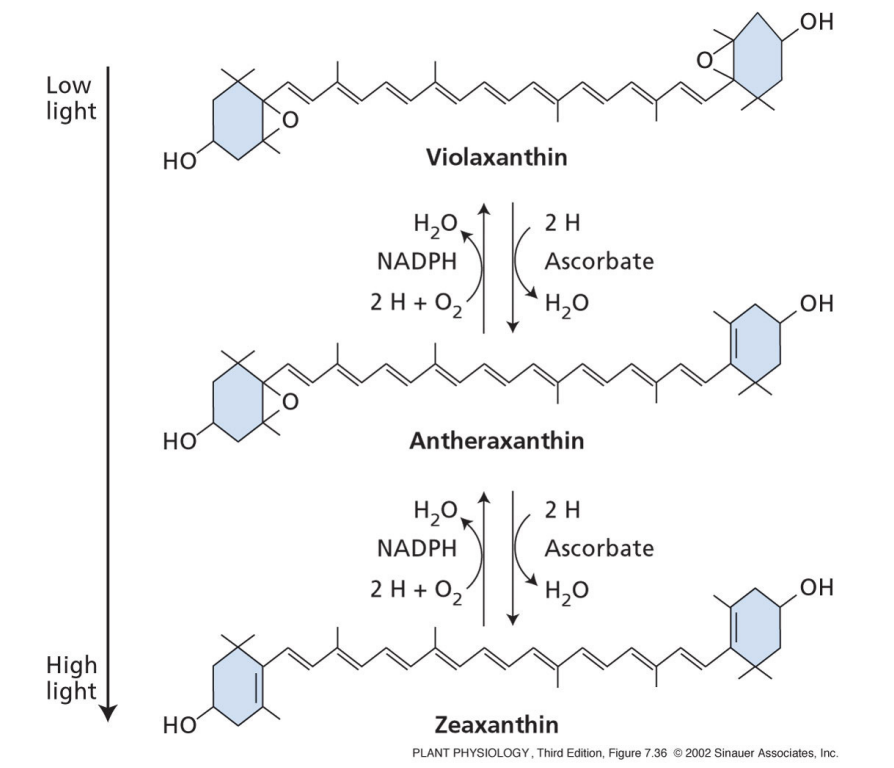
what is non-photochemical quenching?
quenching of chlorophyll fluorescence by processes other than photochemistry
what is the xanthophyll cycle?
in high light
violaxanthin converted to zeaxanthin
allows for dissipation of heat energy
quenched state of PSII = zeazanthin
unquenched state of PSII = violaxanthin
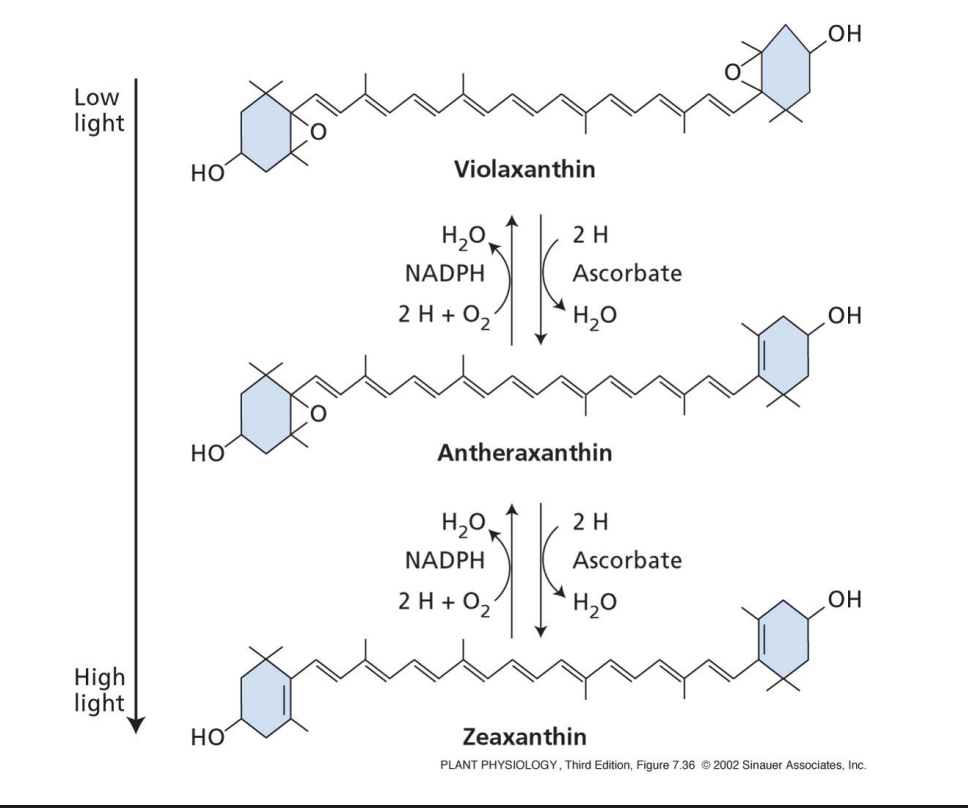
what is photoinhibition?
inhibition of photosynthesis by excess light
D1 membrane protein in PSII is a primary target for photoinhibition
D1 has a high turnover
susceptible to oxidative damage
how does LHCII movement prevent high light damage
when tons of light energy is going towards PSII
reduced plastoquinone accumulates
kinase phosphorylates LHCII
phosphorylation causes LHCII to move out of stacked grana (PSII) to unstacked regions (PSI)
directs absorbed light energy to PSI
prevents creation of oxygen free radicals
how is heat dissipated from leaves?
evaporative heat loss: H2O evaporation through stomata
sensible heat loss: directs heat loss to the air
how does isoprene production dissipate excess heat?
high temperature causes isoprene to be made from terpenes
isoprenes stabilize photosynthetic membranes and are released into the atmosphere
examples of sources
mature leaves
starch
examples of sinks
fruit
flowers
new leaves
roots
characteristics of sieve cells
no nucleus
no ribosomes
no vacuole
no microfilaments
no golgi
modified endoplasmic reticulum
separated by sieve plates with pores
characteristics of companion cells
numerous mitochondria
supply sieve elements with ATP, proteins, and RNA
connected to sieve elements by plasmodesmata
mechanisms for phloem loading
passive/symplastic: cells of mesophyll are connected to phloem and companion cells through plasmodesmata → sugar concentrations are equal in mesophyll and phloem in leaves
polymer trapping: all cells connected by plasmodesmata but not all plasmodesmata have same size exclusion limit, higher order oligosaccharides are made in companion cells → higher order oligosaccharides are trapped, can only move forward into the phloem
active/apoplastic: sieve cells and companion cells are connected by plasmodesmata but not connected to the rest of the mesophyll → requires transmembrane loading, SWEETs and SUCs/SUTs
what is polymer trapping?
all cells connected by plasmodesmata
not all plasmodesmata have the same size exclusion limit
higher order oligosaccharides are synthesized in companion cells
forces sugars to move forward into the phloem, cannot go backward
sucrose + galactose → raffinose
raffinose + galactose → stachyose
sucrose, raffinose, and stachyose are transported into the phloem
what are SWEETs?
sucrose uniporters
passive transport
usually exporters because concentration of sucrose is higher inside the cell than outside