chapter 3: observing microorganisms through a telescope
1/36
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
37 Terms
1 kilometer (km)
1000m
1 decimeter (dm)
1/10m = 0.1m = 10-1m
1 centimeter (cm)
1/100m = 0.01m
1 millimeter (mm)
1/1000m = 0.001m
1 micrometer (um)
1/1,000,000m = 0.000001m = 10-6m
1 nanometer (nm)
1/1,000,000,000m = 0.0000000001m = 10-9m
lenses
focus light rays at a specific place called the focal point
distance b/w center of lens and focal point is the focal length
strength of lens is related to focal length
-shorter the focal length = more magnification
refraction
bending or change in the angle of the light rays as it passed through a medium such as a lens
lenses & the bending of light
light is refracted (bent) when passing from one medium to another
refractive index: a measure of how greatly a substance shows the velocity of light OR a measure of the light-bending ability of a medium
direction & magnitude of bending is determined by the refractive indexes of the two media forming the interface
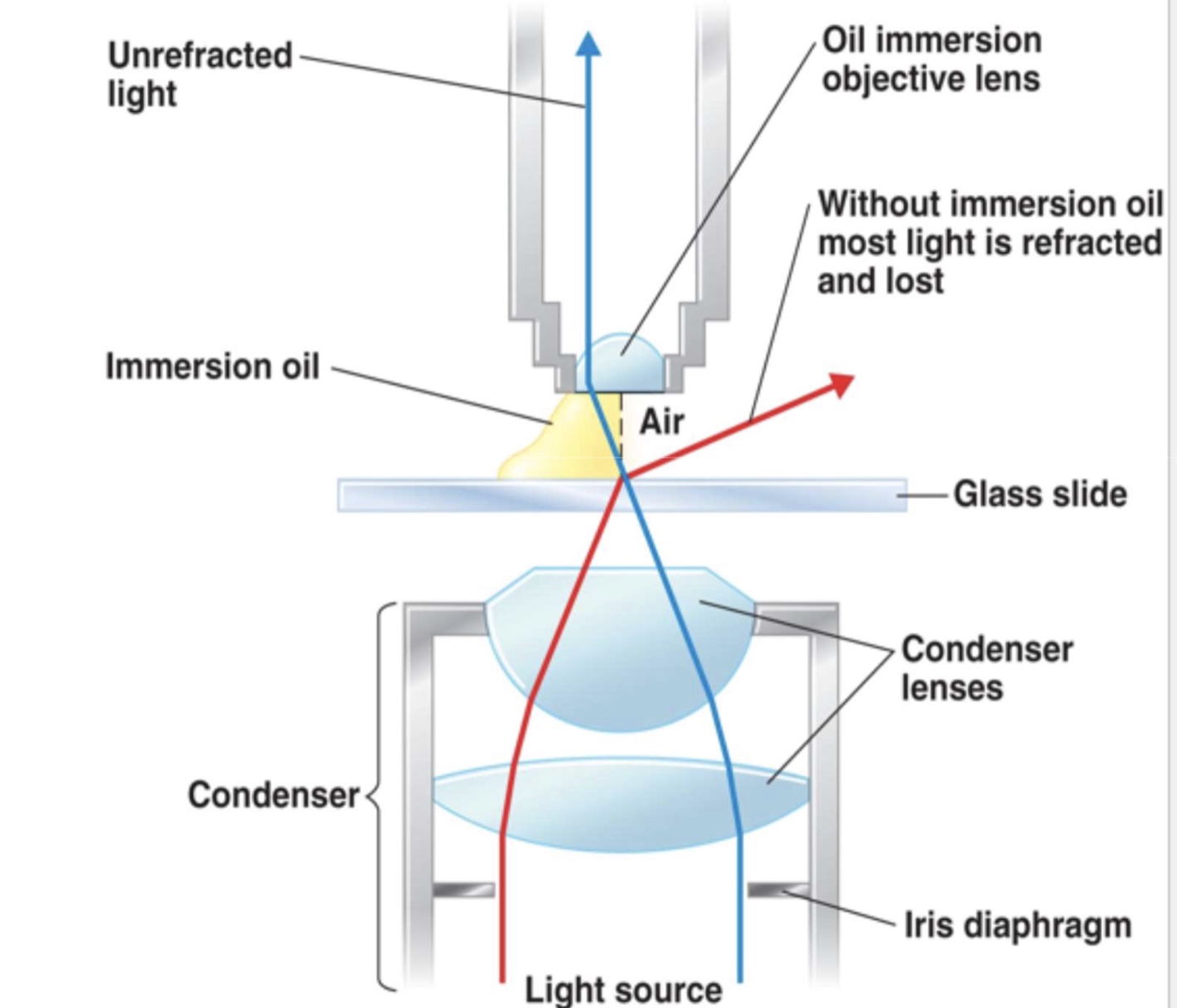
to achieve high magnification, objective lens must be small, but a small lens will lose much of a bending light. so immersion oil is used that has the same refractive index as glass so light will not bend any further
refraction in the compound microscope using an oil immersion objective lens
the light may bend in air so much that is misses the small high-magnification lens
immersion oil is used to keep light from bending
microscope resolution OR resolving power
resolution is the ability of a lens to separate or distinguish small objects that are close together
-microscopic resolution is the shortest distance b/w 2 points in a microscope’s field of view that can still be distinguished as 2 distinct objects
wavelength of light is major factor in resolution as shorter wavelength gives greater resolution
white light has long wavelength and cannot resolve structures less than 0.18micrometers apart
resolution of leeuwenhoek’s microscope was 1 micrometer
resolving power = wavelength of light / 2 x numerical aperture
property magnification
scanning: 4x
low power: 10x
high power: 40-45x
oil immersion: 90-100x
illuminator
the light source
condenser
has lenses that direct rays through the specimen
objective lens
lens closet to the specimen and used to magnify (make object appear enlarged) the image; different powers such as 4X, 10X, 100X
ocular lens or eyepiece
magnifies the image & also used to view the image, magnification power is 10X
light microscope
any kind of microscope that uses visible light to observe specimen
includes:
bright field
dark field
phase contrast
fluorescence
-these are compound microscopes, image formed by action of > or equal to 2 lenses
-microscopes used by leeuwenhoek was a simple microscope, 1 lens.
bright-field microscope
produces a dark image against a brighter background
has several objectives lenses these are parfocal microscopes (remain in focus when objective lenses switched)
total magnification: product of the magnifications of the ocular lens and the objective lens (ocular lens magnification x objective lens magnification)
dark field microscopy
used to study living microorganisms, dark field condenser is used that contains an opaque disc. it will block light from directly entering the objective lens. only the light reflected from the specimen enters the objective lens,
specimen appears light against black background. very thin spirochete as treponema pallidum (causes syphilis) is examined
electron microscopy
used to examine objects smaller than 0.2 micrometers like viruses or internal cellular structures, a beam of electrons is used instead of light
better resolution bcs of shorter wavelength of electron (100,000X shorter than light)
electromagnetic lenses (not glass) are used to focus beam of electrons onto a specimen
images are always black/white but may be colored artificially
two types:
-transmission electron microscope (TEM)
-scanning electron microscope (SEM)
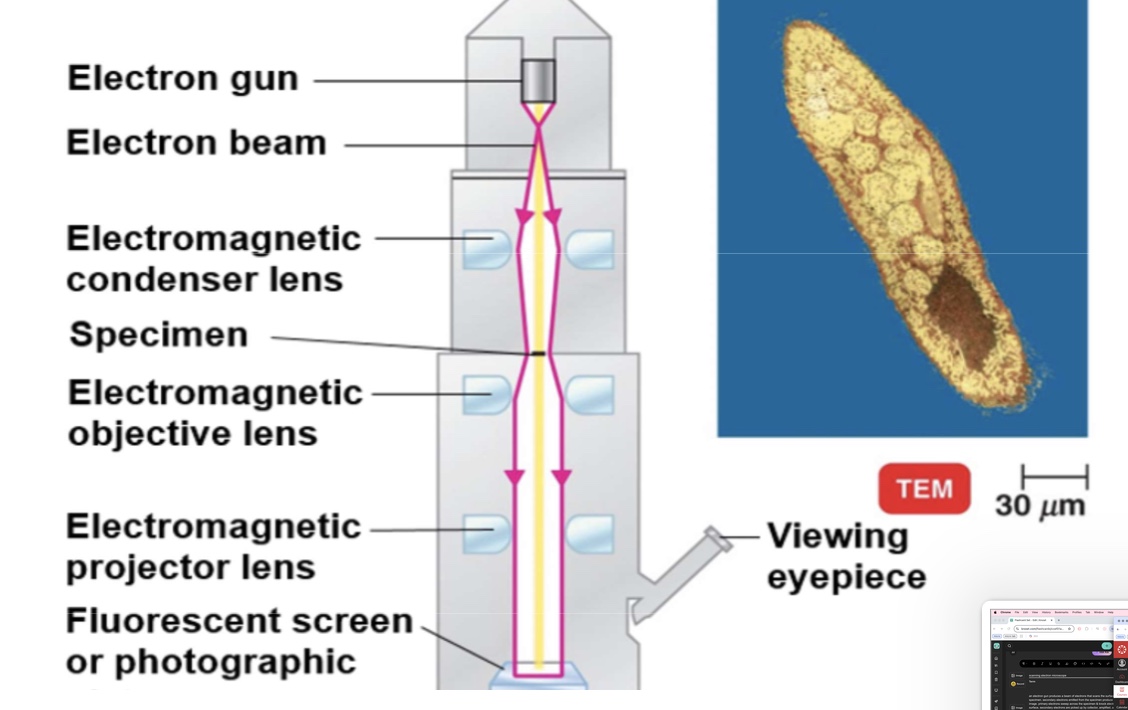
transmission electron microscope
electrons pass through the specimen and are scattered. magnetic lenses focus on image onto a fluorescent screen or photographic plate. (right) this colorized transmission electron micrograph. (TEM) shows a thin slice of paramecium. in this type of microscopy. the internal structures present in the slice can be seen.
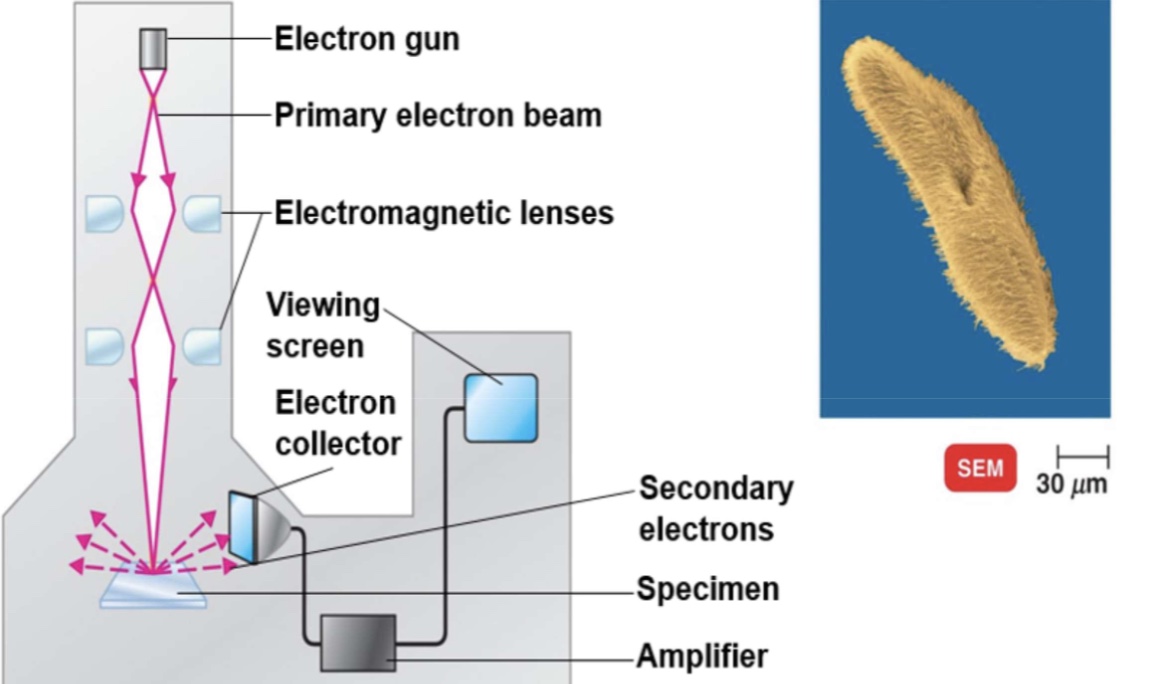
scanning electron microscope
an electron gun produces a beam of electrons that scans the surface of an entire specimen. secondary electrons emitted from the specimen produce a 3 dimensional image. primary electrons sweep across the specimen & knock electrons from its surface, secondary electrons are picked up by collector, amplified, and transmitted onto viewing screen or photographic plate. In this colorized scanning electron micrograph, the surface structures of paramecium are seen. see the 3 dimensional appearanceo of cell, in contrast of 2 dimensional in transmission electron.
TEM electron microscopy
ultra thin section required
objects magnified 10,000 to 100,000X
resolution about 2.5 nm
internal structures seen
electron beam passes through the specimen and produces image
image can be viewed thru eye piece
SEM electron microscopy
no sectioning required
objects magnified 1,000 to 10,000X
resolution abt 20nm
external structures seen
electron beam removes electron from surface of specimen
image is viewed on the viewing screen
smear prep: the smear
a bacterial smear is a fried preparation of bacterial cells on a glass slide, requires only a small amount of the microbial culture. if smear is thick, light may not pass thru smear making it hard to see the morphology of microbial cell
good smears is one in which:
microbes are evenly spread on the surface of slide
microbes are not washed away during staining
microbial forms are not distorted
smear prep: heat fixation
done by passing the air-dried smear several times over flame having smear side up OR by using the blow dryer from the underside of the slide (smear side up)
smear is fixed (attached) on the slide by heat otherwise smear will be washed away during the staining procedure
heat fixation coagulate bacterial proteins so bacteria stick to the slide surface
heat fixation also kills bacteria
chemistry of a stain
chemically, a stain is an organic compound, 3 parts of a stain:
benzene: organic color solvent
chromophore: chemical that is color (it gives color to the organic solvent)
auxochrome: ionizes the chromogen so that it can bind with the cells, fiber, or tissues
chromogen = benzene + chromophore. a colored compound not a stain.
staining
microbes are generally colorless, so it is difficult to visualize them. staining the color the microbes (or background) with a dye that:
creates a contrast b/w the bacteria & the background
emphasizes certain microbial structures
good for study of microbial properties & to group microbes in specific groups for diagnosis
stains are salts composed of a positive & negative ion, one of which is colored & is known as the chromophore
-in a basic dye the chromophore is a CATION (ex. crystal violet, methylene blue, malachite green, safranin)
-in an acidic dye the chromophore is an ANION (ex. esoin, acid fuchsin, nigrosin)
as bacteria are slightly negatively charged, acidic dyes are repelled by most of the bacteria, a dye stains the background (negative staning, heat fixation is not required)
negative staining is used to observe the overall shape, size and capsules (if present)
what are the 3 kinds of staining procedures?
simple
differential
special
simple staining
an aqueous or alcohol solution of a single basic dye, creates a contrast between bacteria & the background
-highlights the entire microorganism, so used to study the cell shape, size, and arrangement
-simple & ez to use
during simple staining:
-smear is heat fixed, simple stain is applied for a certain length of time & washed off, then the slide is dried & studied (ex. methylene blue, crystal violet, carbolfuchsin, safranin)
-sometimes a mordant is also used to hold the stain or coat the specimen to enlarge it.
differential staining
requires the use of at least 3 chemical reagents, that are applied sequentially to a heat fixed smear.
primary stain: imparts its color to all cell
decolorizing agent: based on chemical composition of cellular components, the decolorizing agent may or may not remove the primary stain from the entire cell or only from certain cell structures
counterstain: has contrasting color to that of the primary stain
-following decolorization, if the primary stain is not washed out, the counterstain is not absorbed and the cell or its components will retain the color of the primary stain
gram staining the procedure
developed by hans christian gram in 1884
classify bacteria in 2 groups: gram positive & gram negative
4 steps:
primary stain Crystal violet (basic purple dye) applied to a heat fixed smear
after washing off the primary stain, iodine (mordant) is applied. it will make crystal violet iodine complex (CV-I complex). then mordant is washed too. all bacteria now is dark violet or purple.
alcohol or an acetone solution is used as a decolorizing agent. which removes the purple color (CV-I complex) from gram negative bacteria, but gram POS bacteria retain the PURple. most important step. over decolorizing will have loss of primary stain, causing gram pos to appear gram neg but under-decolorizing will cause gram neg to appear as gram pos.
alcohol is rinsed off, and the slide is now stained with counterstain Safranin (basic red dye), smear washed again, blotted dry & examined microscopically
gram pos bacteria have thick layer of peptidoglycan in cell wall. cv-I complex is not washed off by alcohol so they will retain the complex and stay PURPLE
gram neg bacteria have thin layer of peptidoglycan and also have a layer of lipopolysaccharide as part of their cell wall. alcohol wash disrupts the lipopolysaccharide layer and cv-l complex is washed through the thin layers. so gram neg will stay colorless unless stained with counterstain safranin, after which they are pink.
gram reaction of a bacterium is clinically important as gram pos and gram neg respond to diff antibiotics. beta lactams (ex. pencillins & cephalosporins) are more active against gram pos bacteria and less active against gram negs as they cannot penetrate the lipopolysaccharide later. some bacteria stain poorly or not at all with gram staining (myobacteria)
acid-fast staining
used to distinguish myobacterium species & some species of nocardia. acid fast stain binds strongly to a waxy material in the cell wall.
in the procedure:
carbol fuchsin (red dye) is applied to a fixed smear. heating enhances the penetration of this dye
after cooling & washing w/ water, smear is washed with acid alcohol (decolorizer). non acid fast bacteria lose the primary stain. acid-fast bacteria retain the dye because it is more soluble in the cell wall lipids than in the acid alcohol.
counter stain methylene blue is used which stain non acid-fast bacteria as blue
special staining
used to color specific parts of microorganism
endospore (special resistant, dormant structure)
flagella (used for locomotion)
capsule (gelatinous covering)
special staining: endospore staining
formed by only a few genera
simple stain & gram stain dyes do not penetrate the wall of endospore
schaeffer-fulton endospore stain is used
-heat fixed smear prepared
-primary stain malachite green is applied & heated to steaming for about 5 mins (to help stain penetrate with endospore wall)
-washed w water to remove extra malachite green
-counterstain safranin is applied to stain other parts of the cell as pink
special staining: capsules staining
presence of capsule determine a microbe’s virulence, capsular material is soluble in water, so washing can remove this gelatinous covering
india ink or nigrosin is used to stain background dark
then simple stain like safranin is used to stain cells
capsules do not accept most dye likes safranin
capsules look like halos around stained cells
special staining: flagella staining
flagella are very thin!
a mordant is used to build up the diameter of flagellum & when stained with carbolfuchsin it becomes more visible under the light microscope
# and location of flagella is used in diagnosis