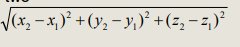Analysing crystals
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
8 Terms
Why is it difficult to locate h-bonds, and light atoms in the presence of heavy atoms when using X-ray diffraction
X-rays are diffracted by electrons, and so atoms with lots of electrons show up better than those with very few
How can we view these atoms with few electrons
We can instead observe the neutrons, these will be diffracted by crystalline solids giving their structural information.
They interact with nuclei rather than electrons so will see the atoms differently
This means that H bonds and light will show up clearly
What are the three factors we use to express structure data
Once solved and refined a crystal structure is expressed in terms of:
• cell parameters (a, b, c; α, β, γ)
• space group (every crystal belongs to one of 230 symmetry descriptions)
• atomic coordinates
In order to visualize a structure or calculate the distances between atoms all there must be taken into account.
What unit do we use for volume
Angstrom
How are the positions of atoms expressed in crystallography, and why this way.
Expressed as fractional co-ordinates as they represent positions as fractions of the unit cells lattice vectors, creating standardised measurements not dependant by specific units of length
During analysis what system must we use for atomic position
Cartesian co-ordinate’s
What is the formula for converting fractional to cartesian coordinates in orthogonal crystal systems
(orthogonal is cubic, tetragonal, and orthorhombic)
Xcart = a * xfrac
ycart = b * yfrac
zcart = c * zfrac
What’s the formula for calculating distances using cartesian coords