HSC Chemistry (Mods 5, 6 & 7)
1/141
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
142 Terms
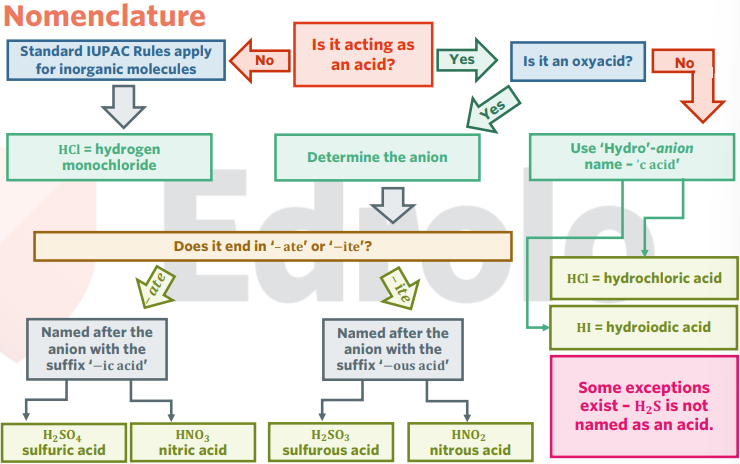
nomenclature of acids
indicators
indicators are weak acid dyes that exist in equilibrium
HIn(aq) <-> H+(aq) + In-(aq)
predict the products of acid reactions and write balanced equations to represent acid/carbonate reactions
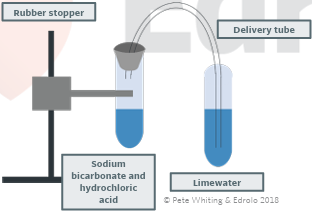
metal carbonate + acid → carbon dioxide gas + salt + water
strength of the reaction depends upon the strength an concentration of the acid
exothermic
presence of carbon dioxide can be confirmed with the limewater test
hydrogen carbonates have the same products
predict the products of acid reactions and write balanced equations to represent acid/metal reactions
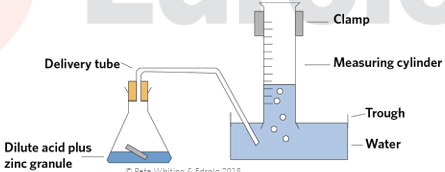
metal + acid → hydrogen gas + salt + energy
strength of the reaction depends upon the strength an concentration of the acid and the position of metals on the activity series
exothermic — if it’s too exothermic (as in the case of group 1 metals), the H gas produced can explode
presence of H gas confirmed with the pop test
applications of neutralisation reactions in everyday life
ant bites
these are formic acid
they can be neutralised with a weak base
applications of neutralisation reactions in industrial processes
Industrial neutralisation of acids and bases requires proton transfer, this is exothermic.
On a large scale this can release large amounts of energy.
This can cause the water in the system to boil, spit, bubble and lift corrosive chemicals up as it bubbles and spits water droplets.
Chemicals can also be neutralised when spills/accidents occur.
An acid or base that is neutralised turns largely to harmless salts and water – but this reaction is exothermic so caution is necessary.
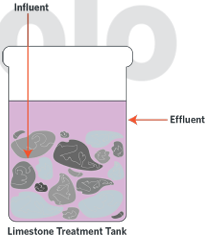
Lime stone (CaCO3) – a basic salt – can be used to neutralise acidic waste
enthalpy of neutralisation
neautralisation is a transfer of protons and proton transfer is always an exothermic process
apply standard enthalpy formula
include the mass of both solutions combined AND the moles of water produced
calculation steps
write the equation
calculate nwater
calculate the heat of neautralisation
calculate the enthalpy of neutralisation

Lavoisier to Davy theories
1776 - Lavoisier was the first to define an acid— as containing oxygen
1810 - Davy discovers various acids that do not contain oxygen, but they all contain hydrogen
Arrhenius’ theory
1884
acids ionise in water to give H+ which will bond to water and create the hydronium ion
bases ionise in water to give OH-
acid + base → salt + water
limitations
only applies to aqueous solutions
only applies to substances that give off those ions
Brønsted–Lowry theory
1923
an acid is a species that donates a proton to a base
a base is a species that accepts a proton from an acid
acid-base reactions involve proton transfer
overcame from Arrhenius:
not necessary to be in aqueous solutions
allows for amphiprotic substances (can act as an acid or a base)
limitations
still requires an acid to have a H+ to donate
pH formula
pH = -log[H+]
pH = 14 - pOH
calculation of pOH
pOH = -log[OH-]
pOH = 14 - pH
calculation of hydrogen ion concentration [H+]
write acid dissociation equation to find mole ratio of acid to H+ ions
find concentration of acid
therefore, find concentration of H+ ions
calculation of hydroxide ion concentration [OH-]
write base dissociation equation to find mole ratio of acid to OH- ions
find concentration of base
therefore, find concentration of OH- ions
write ionic equations to represent the dissociation of acids and bases in water
HA ⇌ H+ + A-
HB ⇌ OH- + B+
conjugate acid/base pairs
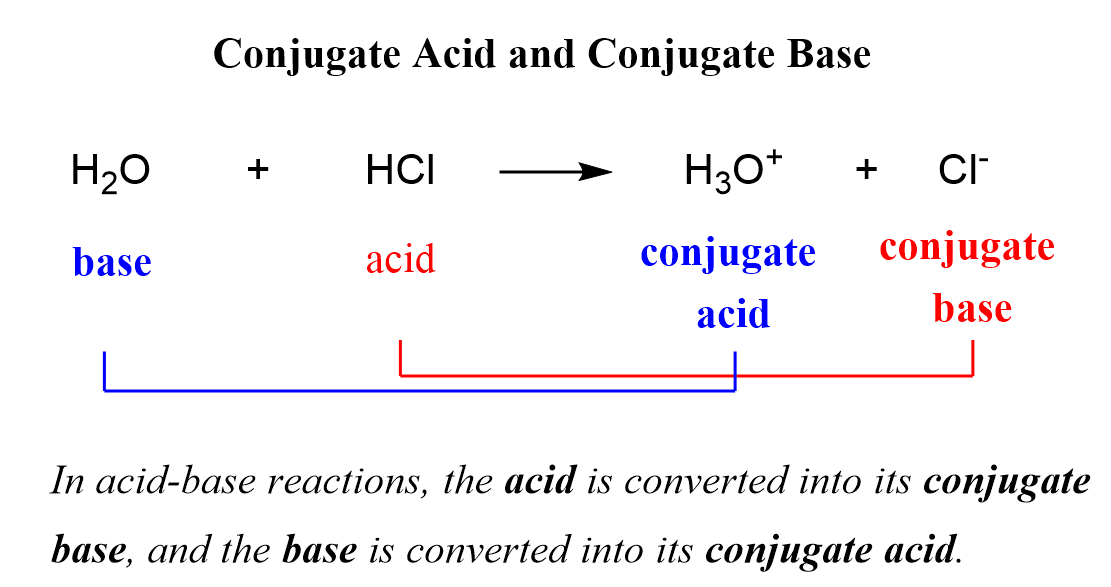
amphiprotic
a substance that can act as both a B-L acid and base (proton donor and acceptor)
sodium hydrogen carbonate
NaHCO3 + HCl ⇌ NaCl + H2O + CO2
potassium dihydrogen phosphate
KH₂PO₄
model the difference between strong/weak acids & concentrated/dilute acids
calculate the pH of the resultant solution when solutions of acids and/or bases are diluted or mixed
write acid/base reaction equation
find number of moles of either acid/base in excess
write the acid/base dissociation equation
use this to find concentration of H+/OH-
use this concentration to find pH/pOH
titration
Volumetric technique where the concentration of an analyte is determined by neutralisation with a standardised chemical.
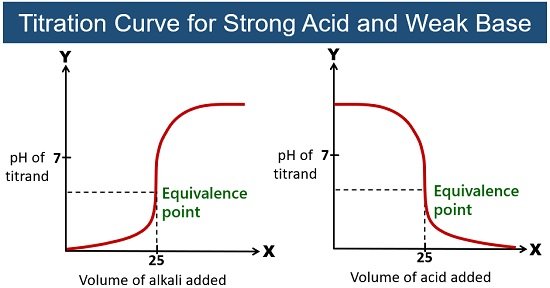
titration curves — strong acid/strong base
As the base is added, OH- ions add to H+ ions to make water and reduce conductivity. As the equivalence point is passed the OH- ions start to build up again and increase solubility. The graph is the same for the reverse reaction.
conductivity graphs

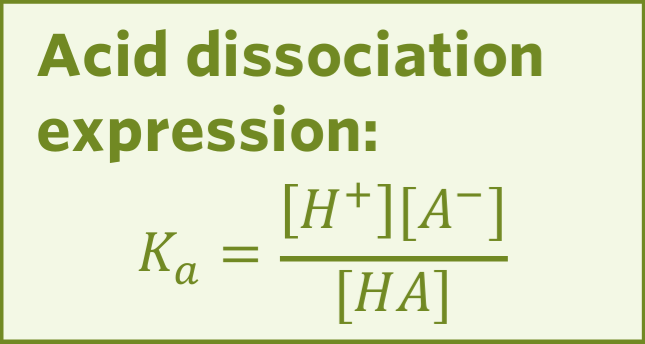
Ka
Ka is a measure of ionisation of an acid.
The larger the Ka the more the acid dissociates and therefore the stronger the acid.
Ka is completely acceptable in expressing the strength for strong acids, however it becomes unmanageable for weak and very weak acids.
This is where pKa becomes useful.
acid/base analysis techniques that are applied in industries
Many industrially produced fertilisers are the products of neutralisation reactions.
These reaction vessels can be monitored using industrial pH probes as a method of knowing when reactions are complete or which reagent needs increasing.
Ammonium nitrate is reacted with nitric acid in a neutraliser. Here the pH is measured to ensure that indeed the correct mixture has been achieved.
Ammonium nitrate has a pH of 5.3 as HNO3 is a strong acid.
Other aspects of this production need to be monitored as NH4NO3 is an extremely effective explosive that will combust without the presence of oxygen (it can use the oxygen present in the nitric oxides).
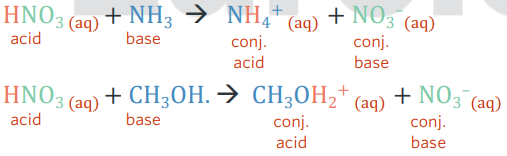
acid/base analysis techniques that are applied by Aboriginal and Torres Strait Islander Peoples
bull ants and bracken fern
Bracken fern (Pteridium esculentum) has alkaline juices in the leaves when crushed.
Bull ants inject painful formic acid as it stings.
The alkali juices of the bracken fern have been known to neutralise the formic acid.
HCOOH(aq) + BOH(aq) → BHCOO(aq) + H2O(l)
acid/base analysis techniques that are applied using digital probes and instruments
probes and digital measurements allow monitoring to be accurate, reliable and precise
require regular calibration
does not alter the analytes chemically
allows industrial processes to be automated or monitored on very large scales

aspirin back titration
Aspirin (CH3COOC6H4COOH) is a very weak acid that cannot be titrated traditionally
However it reacts with excess NaOH (calculate n(NaOH)initial) to produce NaCH3COO(aq) and HOC6H4COONa(aq) and H2O
The remaining NaOH can be titrated with standardised HCl(aq)
We can than calculate how much NaOH was used in the first reaction
Then the moles of aspirin can be calculated.
Then the mass of CH3COOC6H4COOH present in the tablet can be calculated
buffer
Buffer systems are chemical systems that resist changes in [H+] and [OH- ] – change is much less than expected.
Buffer systems consist of a weak acid and its conjugate base or a weak base and its conjugate acid.
HA(aq) ↔ H+ (aq) + A- (aq)
Both the acid and its conjugate base (or vice versa) are in solution in significant amounts.
The acid/base and its salt must be of higher concentrations than the concentration of the added acid/base. The higher the concentration the better the buffering action.
conduct a practical investigation to prepare a buffer and demonstrate its properties
a buffer can be prepared by mixing solutions of:
A weak acid and its salt.
A strong acid and the salt of a weak base.
A weak acid and a strong base.
buffers can be made on purpose or as the result of a titration.
strong acid/strong base reaction
strong acid + strong base → salt + water + energy
HA(aq) + BOH(aq) → AB(aq) + H2O(l) + energy
exothermic, as proton transfer is exothermic
strong acid/weak base reaction
strong acid + weak base → acidic salt
H+(aq) + B(aq) → HB(aq)
a conjugate acid of the weak base is formed — this is a neutralisation as excess H+ are used up but it leaves an acidic solution and does not become neutral — pH < 7
weak acid/strong base reaction
weak acid + strong base → water + conjugate base
HA(aq) + BOH(aq) → H2O(l) + AB(aq)
all H+ ions are used up with OH- ions and the conjugate base of the weak acid is formed
this is a neautralisation reaction as excess OH- ions are used up but it leaves a basic solution and does not become neutral — pH > 7
the conjugate base anion exists in equilibrium with the water to create OH- ions
properties of acids and bases
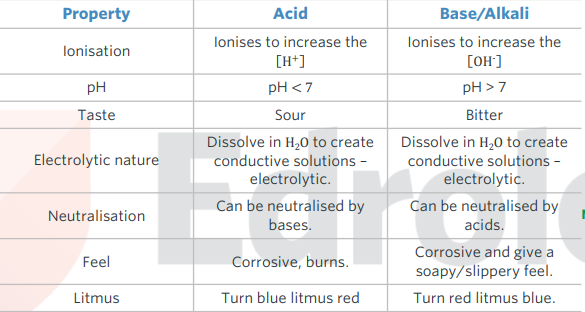
common indicators

volumetric analysis
Any practical analytical technique where the concentrations/volumes /molar quantities are calculated by reacting to known analytes and calculating the known volumes.
primary standard
A standard solution that has a known concentration and is prepared by the chemist.
secondary standard
A solution that has been prepared in the laboratory and has been titrated/ standardised against a primary standard solution.
pKa
pKa = -log(Ka)
back titration
Where a titrand which is unsuitable for titration can be reacted with excess acid or base and the resulting mixture is titrated to see how much acid or base is left over, thus how much was used up
titrand
the solution whose concentration is being determined
titrant
a solution of known concentration that is added to a solution of unknown concentration (the titrand) during a titration, to determine the concentration of the titrand
also known as the analyte
buffer capacity
the amount of acid/base that can be absorbed and still maintain the pH
Buffers are able to absorb the most pH/[H+]/[OH- ] change when:
pKa = pH and
[Acid] = [Conjugate base]
natural buffer systems
blood is a buffered solution at pH 7.35 - 7.45
buffered by H2CO3/NaHCO3 – if pH moves outside of this range, enzymes work less efficiently and metabolism will stop and ultimately end in death
the buffering happens in the equation:
H2CO3(aq) ↔ HCO3 - (aq) + H+ (aq)
By having H2CO3 present in solution with HCO3 - (present as NaHCO3):
As an acid enters the system, the H+ ions are absorbed by the reserve of HCO3 - ions and pH is maintained.
As bases enter the system the H2CO3 ion dissociates, donates a H+ ion to the base (making a salt or water) and pH is maintained.
As CO2 enters the blood as a result of respiration, it moves the two equilibria to the right, the bicarbonate reserves soak up the extra H+ ions.
Excess H2CO3(aq) can reverse the reaction back to CO2(g) and H2O(l) to be exhaled and excess HCO3 - (aq) can be removed via the kidneys to keep [acid] ≈ [base].
cobalt(II) chloride hydrated and dehydrated
Hydrated cobalt ion displays a pink colour whilst cobalt (II) chloride (dehydrated) displays a blue colour. This chemical equilibrium can be easily monitored by the colour change between pink and blue.
Pressure and volume do not affect this reaction as there are no gaseous reactants or products.
Adding HCl to the solution will initially dilute the solution and make the colour paler. The addition also increases [Cl] which in turn increases the rate of forward reaction and shifts the chemical equilibrium to the right. The solution turns blue.
Adding silver nitrate (AgNO3): Ag+ forms a white AgCl precipitate with Cl which decreases [Cl]. The solution quickly turns cloudy white. This change increases the rate of reverse reaction and shifts the chemical equilibrium to the left. Solution turns pink. The pink colour will only be visible after the white precipitate settles to the bottom.
Adding distilled water (de-ionised water) increases the volume of the solution, and more importantly increases the amount of H2O in the system. This initially dilutes the solution, making the colour paler. As a result of this, a dilution effect will occur where the addition of solvent will increase the effective volume of the solution. By Le Chatelier's principle, an increase in the volume of the system will shift the chemical equilibrium to the side with more moles (the left side of the equation since H2O does not have a concentration). Solution turns light pink.
Heating the solution in a water bath drives the forward reaction (endothermic). This increases the concentration of hydrated form of cobalt ion. Solution turns blue.
Cooling the solution in ice drives the reverse reaction (exothermic). This increases the concentration of dehydrated form of cobalt ion. Solution turns pink.
![<ul><li><p>Hydrated cobalt ion displays a pink colour whilst cobalt (II) chloride (dehydrated) displays a blue colour. This chemical equilibrium can be easily monitored by the colour change between pink and blue.</p></li><li><p>Pressure and volume do not affect this reaction as there are no gaseous reactants or products.</p></li><li><p>Adding HCl to the solution will initially dilute the solution and make the colour paler. The addition also increases [Cl] which in turn increases the rate of forward reaction and shifts the chemical equilibrium to the right. The solution turns blue.</p></li><li><p>Adding silver nitrate (AgNO3): Ag+ forms a white AgCl precipitate with Cl which decreases [Cl]. The solution quickly turns cloudy white. This change increases the rate of reverse reaction and shifts the chemical equilibrium to the left. Solution turns pink. The pink colour will only be visible after the white precipitate settles to the bottom.</p></li><li><p>Adding distilled water (de-ionised water) increases the volume of the solution, and more importantly increases the amount of H2O in the system. This initially dilutes the solution, making the colour paler. As a result of this, a dilution effect will occur where the addition of solvent will increase the effective volume of the solution. By Le Chatelier's principle, an increase in the volume of the system will shift the chemical equilibrium to the side with more moles (the left side of the equation since H2O does not have a concentration). Solution turns light pink.</p></li><li><p>Heating the solution in a water bath drives the forward reaction (endothermic). This increases the concentration of hydrated form of cobalt ion. Solution turns blue.</p></li><li><p>Cooling the solution in ice drives the reverse reaction (exothermic). This increases the concentration of dehydrated form of cobalt ion. Solution turns pink.</p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/8082b8d2-9751-4c42-97d0-5d2f83544348.png)
iron(III) nitrate and potassium thiocyanate
Fe(NO3)3(aq) + KSCN(aq) ⇌ FeSCN2+(aq) + KNO3(aq) ΔH<0
net ionic equation: F
e3+(aq) + SCN-(aq) ⇌ FeSCN2+(aq) ΔH<0
Fe3+ is yellow and FeSCN2+ is blood red.
Potassium ion and nitrate ion are spectator ions and are not part of the chemical equilibrium.
Pressure and volume do not affect this reaction as there are no gaseous reactants nor products.
concentration
Addition of Na2HPO4: hydrogen phosphate ions react with Fe3+ to form complex ions:
Fe3+(aq)+HPO42−(aq)→FeHPO4+(aq)
This reduces [Fe3+] which in turn increases the rate of backward reaction and shifts the chemical equilibrium to the left. Solution turns less blood red, paler yellow or colourless.Addition of KSCN crystals increases concentration of reactants of forward reaction. This increases the rate of forward reaction and shifts the equilibrium to the right. Solution turns more blood red.
Addition of Fe(NO3)3 increases the concentration of reactants available for the forward reaction. This will initially make the solution more yellow as [Fe3+] increases. However, by Le Chatelier’s principle, this change in turn increases the rate of forward reaction and shifts the equilibrium to the right. Solution turns more blood red.
Addition of SnCl2 (or any form of Sn2+) reduces Fe3+ to Fe2+. Reduction of Fe3+ is possible because Sn2+ is a stronger reductant.
Sn2++2Fe3+→Sn4++2Fe2+
Reduction decreases [Fe3+] which in turn increases the rate of reverse reaction and shifts the chemical equilibrium to the left. Solution turns less red and more yellow.Adding silver nitrate (AgNO3): Ag+ forms white precipitate with SCN- which decreases [SCN-]:
Ag+(aq)+SCN−(aq)→AgSCN(s)
This increases the rate of backward reaction and shifts the chemical equilibrium to the left. Solution turns cloudy white. Precipitate will settle to the bottom of the tube if left undisturbed for a long time.
temperature
The formation of iron (III) thiocyanate complex ion is an exothermic reaction. This means the forward reaction is favoured at a low temperature.
When the solution is placed in ice-cold water, more iron thiocyanate ion is formed. This causes the solution to appear more blood red.
As the temperature of water increases, the reverse reaction becomes more favoured. This causes the solution to appear less blood red and start to adopt a yellow colour.
![<p><em>Fe(NO<sub>3</sub>)<sub>3(aq)</sub> + KSCN<sub>(aq)</sub> </em>⇌ FeSCN<sup>2+</sup><sub>(aq)</sub> + KNO<sub>3(aq)</sub> <span>ΔH<0</span><br></p><p>net ionic equation: F</p><p>e<sup>3+</sup><sub>(aq)</sub> + SCN<sup>-</sup><sub>(aq)</sub> <span>⇌ </span> FeSCN<sup>2+</sup><sub>(aq)</sub> ΔH<0</p><ul><li><p>Fe<sup>3+</sup> is <span>yellow</span> and FeSCN<sup>2+</sup> is <span>blood red</span>.</p></li></ul><ul><li><p>Potassium ion and nitrate ion are spectator ions and are not part of the chemical equilibrium.</p></li></ul><ul><li><p>Pressure and volume do not affect this reaction as there are no gaseous reactants nor products.</p></li></ul><p><u>concentration</u></p><ul><li><p>Addition of Na<sub>2</sub>HPO<sub>4</sub>: hydrogen phosphate ions react with Fe<sup>3+</sup> to form complex ions:</p><p><span>Fe<sub>3</sub><sup>+</sup><sub>(aq)</sub>+HPO<sub>4</sub><sup>2−</sup><sub>(aq)</sub>→FeHPO<sub>4</sub><sup>+</sup><sub>(aq)</sub><br></span>This reduces [Fe<sup>3+</sup>] which in turn increases the rate of backward reaction and shifts the chemical equilibrium to the left. Solution turns less blood red, paler <span>yellow</span> or colourless.</p></li><li><p><span>Addition of KSCN crystals increases concentration of reactants of forward reaction. This increases the rate of forward reaction and shifts the equilibrium to the right. Solution turns more blood red. </span></p></li><li><p><span>Addition of Fe(NO</span><sub>3</sub><span>)</span><sub>3</sub><span> increases the concentration of reactants available for the forward reaction. This will initially make the solution more yellow as [Fe</span><sup>3+</sup><span>] increases. However, by Le Chatelier’s principle, this change in turn increases the rate of forward reaction and shifts the equilibrium to the right. Solution turns more blood red.</span></p></li><li><p>Addition of SnCl<sub>2</sub> (or any form of Sn<sup>2+</sup>) reduces Fe<sup>3+</sup> to Fe<sup>2+</sup>. Reduction of Fe<sup>3+</sup> is possible because Sn<sup>2+ </sup>is a stronger reductant.<br><span>Sn<sup>2+</sup>+2Fe<sup>3+</sup>→Sn<sup>4+</sup>+2Fe<sup>2+</sup></span><br>Reduction decreases [Fe<sup>3+</sup>] which in turn increases the rate of reverse reaction and shifts the chemical equilibrium to the left. Solution turns less red and more yellow.</p></li><li><p>Adding silver nitrate (AgNO<sub>3</sub>): Ag<sup>+</sup> forms white precipitate with SCN<sup>-</sup> which decreases [SCN<sup>-</sup>]:</p><p><span>Ag<sup>+</sup><sub>(aq)</sub>+SCN<sup>−</sup><sub>(aq)</sub>→AgSCN<sub>(s)</sub></span><br>This increases the rate of backward reaction and shifts the chemical equilibrium to the left. Solution turns<strong> cloudy white.</strong> Precipitate will settle to the bottom of the tube if left undisturbed for a long time.</p></li></ul><p><u>temperature</u></p><ul><li><p><span>The formation of iron (III) thiocyanate complex ion is an exothermic reaction. This means the forward reaction is favoured at a low temperature.</span></p></li><li><p>When the solution is placed in ice-cold water, more iron thiocyanate ion is formed. This causes the solution to appear more blood red.</p></li><li><p>As the temperature of water increases, the reverse reaction becomes more favoured. This causes the solution to appear less blood red and start to adopt a yellow colour.</p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/9827cce0-3ab6-4342-a3dc-607912ef3e77.png)
burning magnesium
2Mg(s) + O2(g) → 2MgO(s) ΔH < 0 (combustion)
Flame (heat energy) is required to overcome the relatively small activation energy barrier of the reaction.
Burning magnesium goes to completion (until magnesium strips are fully converted into magnesium oxide). It is an irreversible reaction because magnesium oxide is very stable at standard conditions. The activation energy of the reverse reaction (endothermic) is also much larger.

burning steel wool
4Fe(s) + 3O2(g) → 2Fe2O3(s) ΔH<0 (combustion)
Similar to magnesium, burning steel wool (made of mostly iron) is an example of exothermic, irreversible reaction
The activation energy of the reverse reaction is relatively large under standard conditions.

differences between static and dynamic equilibrium
static equilibrium: reaction has gone to completion (irreversible)
dynamic equilibrium: reaction will not go to completion and con proceed in both the forward and reverse directions (reversible)
open systems
both energy and matter are able to be lost or gained from the environment
e.g. open test tube
equilibrium can NOT be achieved in an open system
closed systems
only energy, NOT MATTER, is able to be transferred to the environment
e.g. test tube with a stopper
equilibrium can only be achieved in a closed system
combustion reactions (enthalpy and entropy)
non-equilibrium system
enthalpy (ΔH) = -ve
entropy (ΔS) = +ve
Gibbs free energy (∆G) = > 0
photosynthesis (enthalpy and entropy)
6CO2(g) + 6H2O(l) → C6H12O6(s) + 6Os(g) ΔH > 0
Photosynthesis is a biological process which requires enzymes (biological catalysts) to take place. These enzymes only catalyse the forward reaction.
The reverse reaction of photosynthesis does not take place because the activation energy is too high in the absence of a suitable enzyme.
While photosynthesis as a chemical equation is the reverse of aerobic respiration, the biological process is distinctively different. The synthesis of glucose from carbon dioxide and water is a highly unfavourable process because it is endothermic and decreases in entropy. It is not possible as a single-step process.
Takes place in an open system within plant cells.
enthalpy (ΔH) = +ve
entropy (ΔS) = -ve
Gibbs free energy (∆G) = > 0
relationship between collision theory and reaction rate in order to analyse chemical equilibrium reactions
example of 2NO2(g) ⇌ N2O4(g)
Molecules of N2O4 can decompose to produce twice as many NO2.
As the N2O4 molecules decompose to produce NO2, their concentration decreases and so does their collision rate. This in turn reduces the rate of the forward reaction.
As NO2 molecules form, they can also collide and result in a reaction to re-form N2O4. This is represented by the reverse reaction.
In the beginning, the forward reaction rate is greater than the reverse reaction rate as there are far more N2O4 than NO2 molecules. This results in a net decrease in [N2O4] and a net increase in [NO2]
However, as the reaction proceeds, the rate of the forward reaction gradually decreases, and the rate of the reverse reaction gradually increases.
This continues until the rates of the forward and reverse reaction become equal, where equilibrium is established. At this point, forward and reverse reactions still occur due to the collision between molecules but no changes in concentration are observed.
equilibrium
a system is at equilibrium when the rate of the forward reactions equals the rate of the reverse reaction
collision theory and the three factors it depends on
Collision theory is a theory which states chemical reactions are the result of collisions between molecules or atoms.
Rate of collision between molecules. This is the frequency at which molecules collide. Greater rate of collision leads to greater reaction rate.
Activation energy of the reaction. For a reaction to occur, the reactants must overcome a certain amount of activation energy. Even if the collision rate is high, if the particles don’t have enough energy reaction will still not occur.
Molecular orientation of reactants. Rate of reaction can increase if reactants collide in the right orientation. Conversely, rate can decrease if the orientation of molecules is not favourable for the formation of product(s).
what factors affect collision theory
Concentration of particles/molecules. A higher concentration means there are more particles moving about in a given volume, increasing the frequency at which particles collide.
Pressure/volume. Changes in pressure and volume affect the collision rate between gaseous particles as they occupy the most volume. An increase in pressure (or reduction in volume) increases the rate of collision.
Temperature is the measurement of the average kinetic energy in a system. A higher temperature (greater energy) means particles move about quicker at greater kinetic energy. This in turn increases the collision rate between them.
what factors affect activation energy
Catalysts reduce the activation energy of a reaction, resulting in an increased rate.
molecular energy distribution
In addition to increasing the collision rate, a higher temperature also allows more collisions to result in a chemical reaction (more successful collisions). This is because, at a higher temperature, more molecules have energy greater than the activation energy to react. This causes the Maxwell-Boltzmann energy distribution graph to shift to the right, without changing the activation energy.
Catalysts reduce the activation energy of a reaction. This does not affect the energy distribution of molecules as it does not change the energy inside the system. However, by reducing the activation energy, more molecules have enough energy to result in a chemical reaction when collisions occur.
In both cases (higher temperature and adding a catalyst), the frequency of a successful collision (one that results in a chemical reaction) increases, and so does the reaction rate.
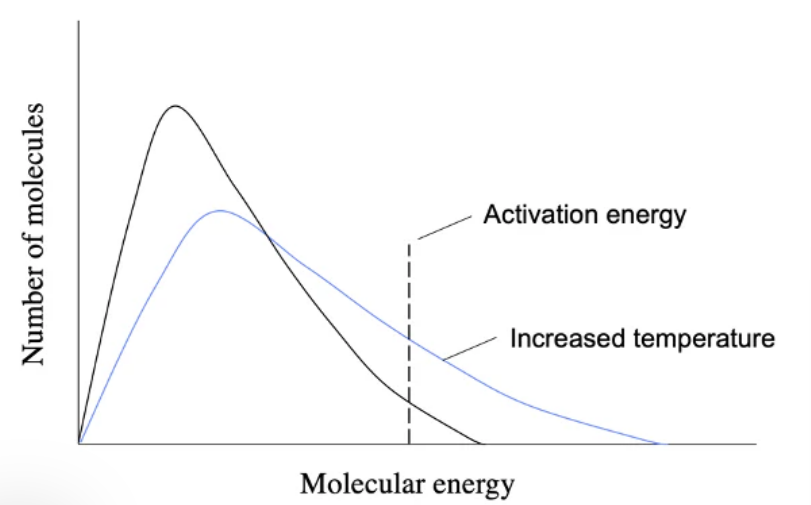
Le Chatelier’s Principle
when a stress is applied to a system at equilibrium, the equilibrium position will move to minimise that stress
interaction between nitrogen dioxide and dinitrogen tetroxide
2NO2(g) ⇌ N2O4(g) ΔH=−58kJmol−1
investigated in a gas syringe
Nitrogen dioxide (NO2) is brown in colour while dinitrogen tetroxide (N2O4) is colourless
pressure and volume
can be tested by pushing or pulling the plunger of the syringe to increase and decrease pressure respectively. Increasing the pressure directly increases the collision rate between gas molecules. This in turn increases the rate of reaction.
Increasing the pressure on the system favours the reaction that would produce less moles of gases. Chemical equilibrium shifts to the side with less moles of gases (N2O4) and solution turns less brown.
Reducing the pressure on the system favours the reaction that would produce more moles of gases. Chemical equilibrium shifts to the side with more moles of gases (NO2) and solution turns more brown.
temperature
Heating the solution in warm water turns it brown as the formation of NO2 is endothermic.
Icing the solution turns it pale as the formation of N2O4 is exothermic.

Keq formule
Keq = [C]c x [D]d/[A]a x [B]b
where
aA + bB ⇌ cC + dD
qualitatively analyse the effect of temperature on the value of Keq
temperature is the only factor which can affect the value of Keq
the effect depends on whether the forward reaction is endothermic or exothermic.
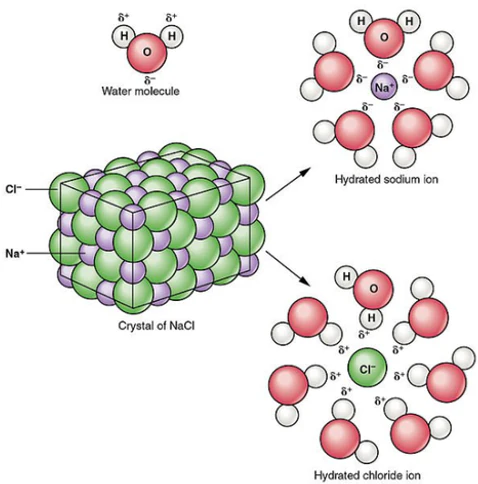
describe and analyse the processes involved in the dissolution of ionic compounds in water
Separation of solvent molecules is endothermic as energy is absorbed to overcome intermolecular forces present between molecules. Hydrogen bonds, dipole-dipole forces and dispersion forces are present between water molecules. Each water molecule can form up to four hydrogen bonds, causing it to have a relatively high boiling point for a molecule of its size.
Dissociation of solutes (ions) is endothermic as energy is absorbed to overcome the electrostatic forces present between ions in the lattice structure of the ionic compound.
Hydration of ions: dissociated ions are surrounded by separated solvent molecules. Intermolecular forces are formed between solutes and solvents, releasing energy in the process. Hydration is an exothermic process.
change of enthalpy during dissolution
The overall change in enthalpy of dissolution of ionic compounds accounts for the change in enthalpy of each of the three steps of dissolution: separation of solvent molecules, dissociation of ionic lattice structure and hydration of free ions.
Dissolution is endothermic if the energy absorbed during solvent separation and dissociation is greater than what is released during ion hydration.
An endothermic dissolution would decrease the temperature of the surrounding environment. Thus, the beaker in which the dissolution occurs will feel colder.
Dissolution is exothermic if the energy absorbed during solvent separation and dissociation is less than what is released during ion hydration.
An exothermic dissolution would increase the temperature of the surrounding environment. Thus, the beaker in which the dissolution occurs will feel warmer.
toxins in cycad fruit — investigate the use of solubility equilibria by Aboriginal and Torres Strait Islander Peoples when removing toxicity from foods
cycad seeds are often used to make bread
they contain cycasin, which can cause cancer and damage to the nervous system
cycasin is soluble in water, therefore it can be removed via leeching
the cycasin in the cycad seed will dissolve in water until the saturation point and reach dynamic equilibrium
running water is used to prevent the establishment of an equilibrium and allow more cycasin to be removed from the seeds
solubility rules
always soluble
NAG SAG
Nitrates (NO3-)
Acetates (C2H3O2-)
Group 1 metals
Sulfates (SO42-)
Ammonium (NH4+)
Group 17 halogens
exceptions
PMS (exception to group 17 & sulfates)
lead
mercury
silver
CaStro Bar (exception to sulfates)
calcium
strontium
barium
potassium chloride and silver nitrate — conduct an investigation to determine solubility rules, and predict and analyse the composition of substances when two ionic solutions are mixed
AgNO3(aq) + KCl(aq) → AgCl(s) + KNO3(aq)
net ionic equation: Ag+(aq) + Cl-(aq) ⇌ AgCl(s)
silver chloride is insoluble in water and will precipitate out of the solution, which is a clear sign that a chemical reaction has occurred

potassium iodide and lead nitrate — conduct an investigation to determine solubility rules, and predict and analyse the composition of substances when two ionic solutions are mixed
Pb(NO3)2(aq) + 2KI(aq) → PbI2(s) + 2KNO3(aq)
net ionic equation: Pb+2 + 2I– ⇌ PbI2(s)
lead iodide is insoluble in water and will precipitate out of the solution, which is a clear sign that a chemical reaction has occurred

sodium sulfate and barium nitrate — conduct an investigation to determine solubility rules, and predict and analyse the composition of substances when two ionic solutions are mixed
Ba(NO3)2(aq) + Na2SO4(aq) → BaSO4(s) + NaNO3(aq)
net ionic equation: Ba2+(aq) + SO42-(aq) ⇌ BaSO4(s)
barium sulfate is insoluble in water and will precipitate out of the solution, which is a clear sign that a chemical reaction has occurred

Ksp formula
Ksp = [C]c x [D]d
(there is no fraction because convention says the solid is always on the left of the arrow, and solids are not included in the equation at all)
calculate the solubility of an ionic substance from its Ksp value
write the solubility equation
PbI2(s) < - > Pb2+(aq) + 2I-(aq)
write the solubility product expression
Ksp = [Pb2+][I-]2
let s be the solubility of the compound
substitute s into the Ksp expression
9.8 × 10-9 = [s][2s]2
solve for s
predict the formation of a precipitate given the standard reference values for Ksp
if Q is higher than Ksp, a precipitate will form.
lattice energy
Ionic compounds occupy a lattice structure, held together by electrostatic forces between cations and anions. Dissolution of ionic compounds requires energy equal to the lattice energy.
Lattice energy indicates bond strength of an ionic compound. It is defined as the energy released through the formation of an ionic compound from its constituent ions.
Lattice energy is dependent on two main factors:
Radius of ions. Larger the ions, lower the lattice energy. For example, KCl has lower lattice energy than NaCl because potassium ion is larger than sodium ion in terms of ionic radius.
Charge of ions. Greater the charge, higher the lattice energy. For example, MgCl2 has greater lattice energy than NaCl because magnesium ion has a +2 charge while sodium ion has a +1 charge.
solubility
the maximum amount of ionic compound that can be added to a given volume of solvent without producing a precipitate
common ion effect
refers to the decrease in solubility of an ionic precipitate by the addition to the solution of a soluble compound with an ion in common with the precipitate
i.e.
suppose some amount of barium sulfate is dissolved in water and reaches a saturated state
if we add sodium sulfate, the concentration of the sulfate ion increases
according to LCP, the equilibrium position will shift to the left to reduce [sulfate ion]
this will also decrease the [barium ion], as these two ions lay on the right of the solubility equation
therefore, adding the common sulfate ion reduced the solubility of the barium sulfate
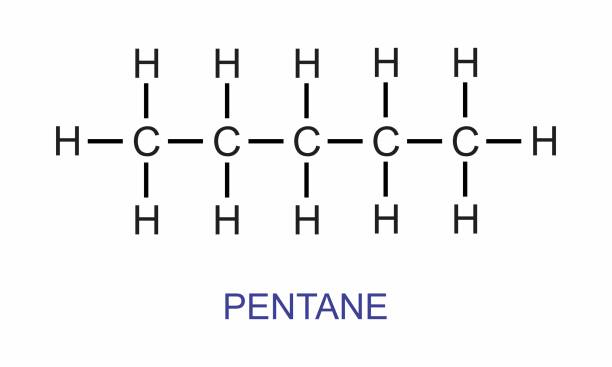
alkanes
saturated hydrocarbon; connected by only single bonds, e.g. pentane
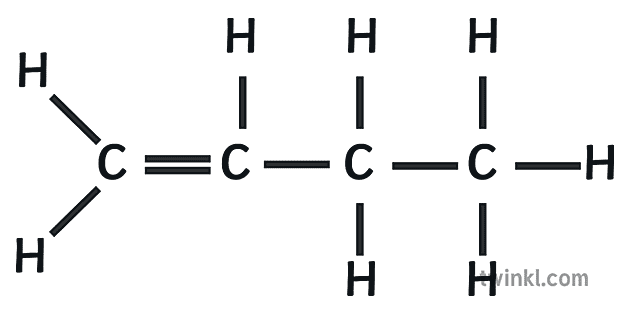
alkenes
unsaturated hydrocarbon; connected by at least one double bond, e.g. 1-butene
alkynes
unsaturated hydrocarbon; connected by at least one triple bond, e.g. 2-pentyne
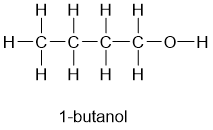
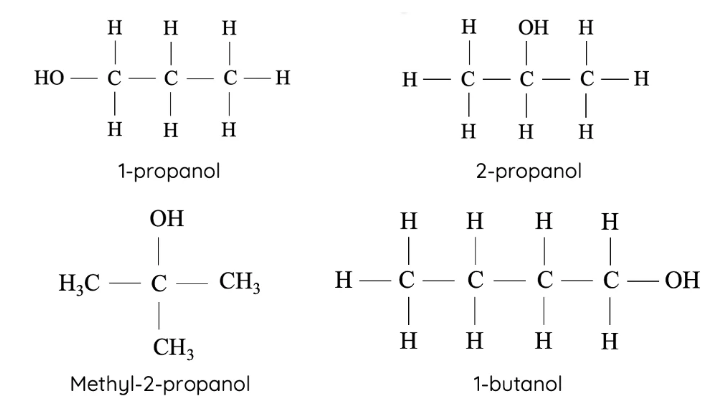
primary alcohols
hydroxyl group (OH) attached to a TERMINAL carbon, e.g. 1-butanol
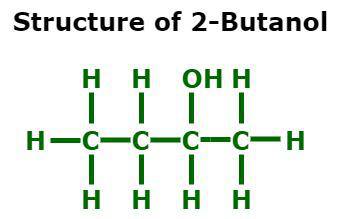
secondary alcohols
hydroxyl group (OH) attached to a carbon which is attached to TWO OTHER carbons, e.g. 2-butanol
tertiary alcohols
hydroxyl group (OH) attached to a carbon which is attached to THREE other carbons , e.g. 2-methyl-2-propanol
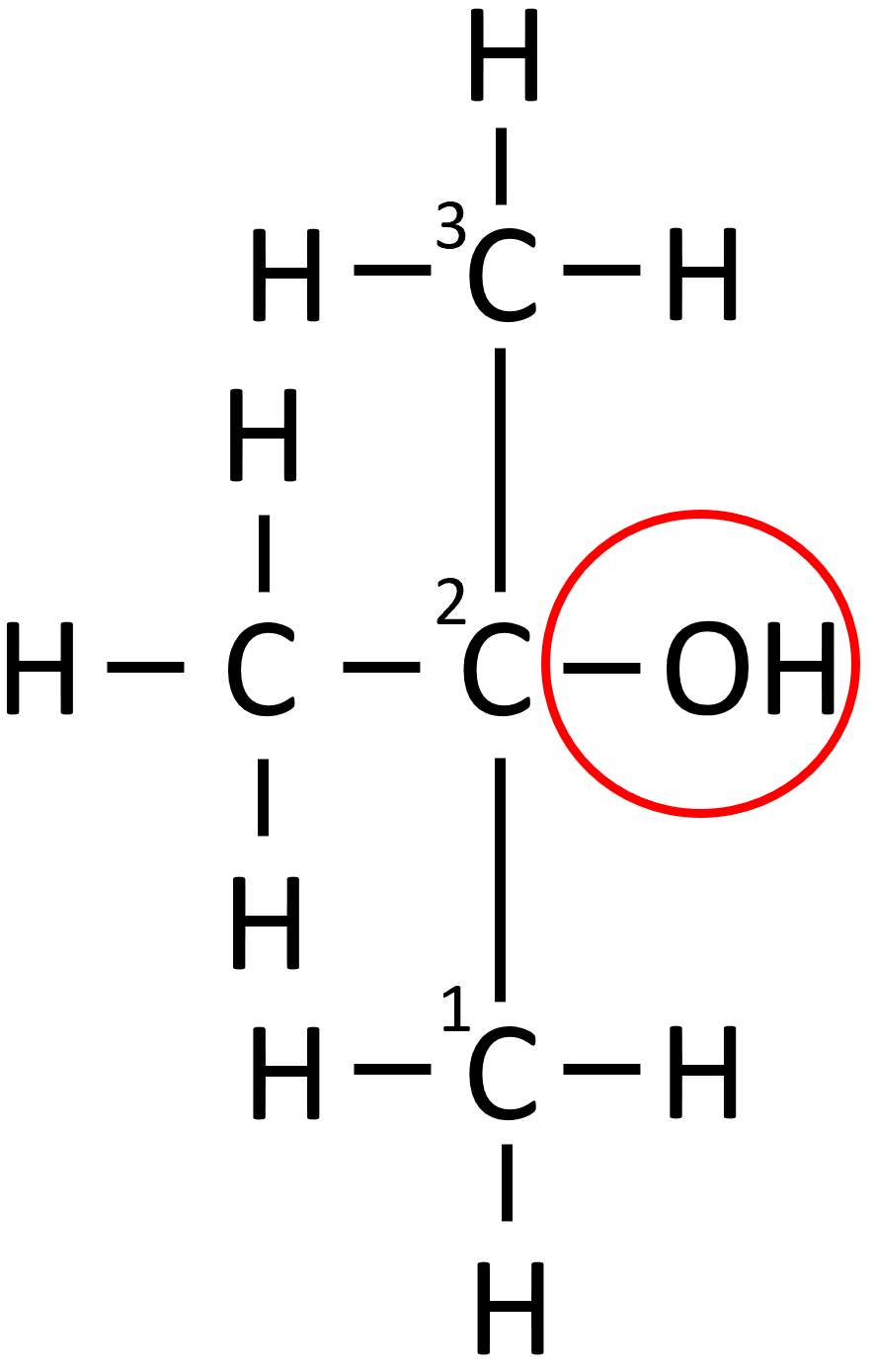
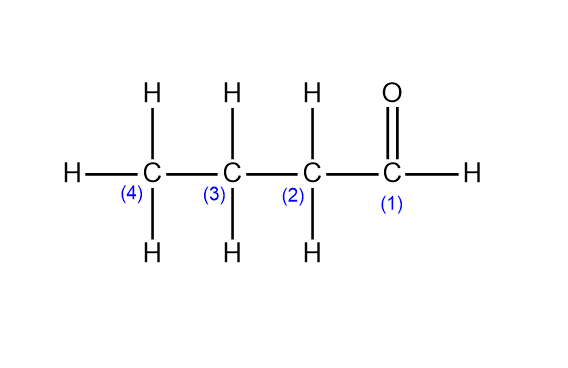
aldehydes
carbonyl group (=O) attached to a TERMINAL carbon, e.g. butanal
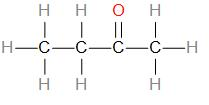
ketones
carbonyl group (=O) attached to a non-terminal carbon, e.g. butanone
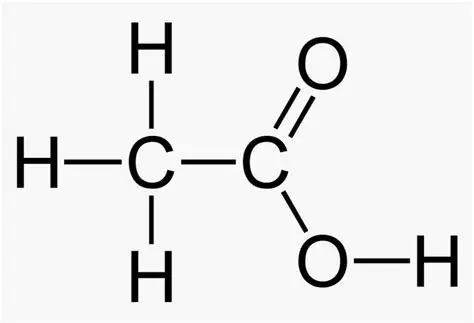
carboxylic acids
a carbonyl group (=O) AND a hydroxyl group (OH) attached to a terminal carbon, e.g. ethanoic (acetic) acid
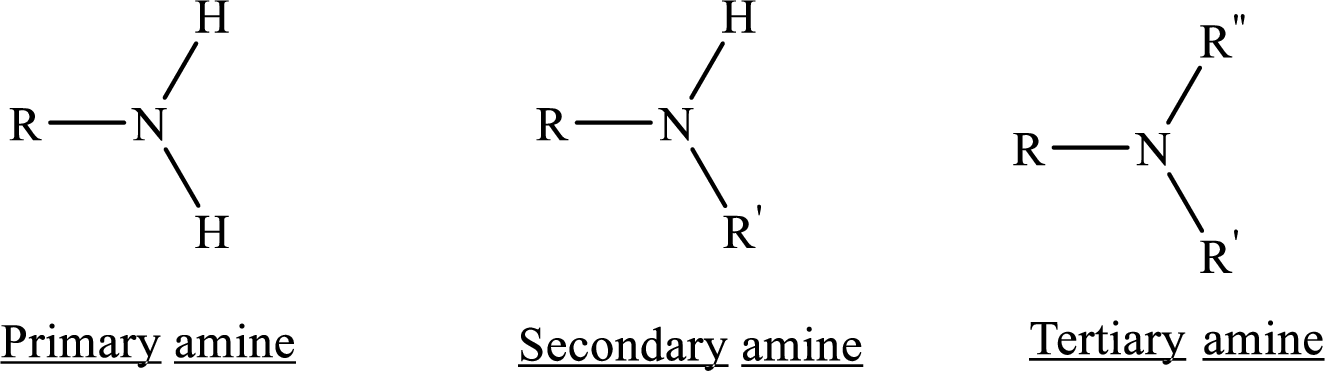
amines
nitrogen with a lone pair of electrons. can be primary, secondary, or tertiary.
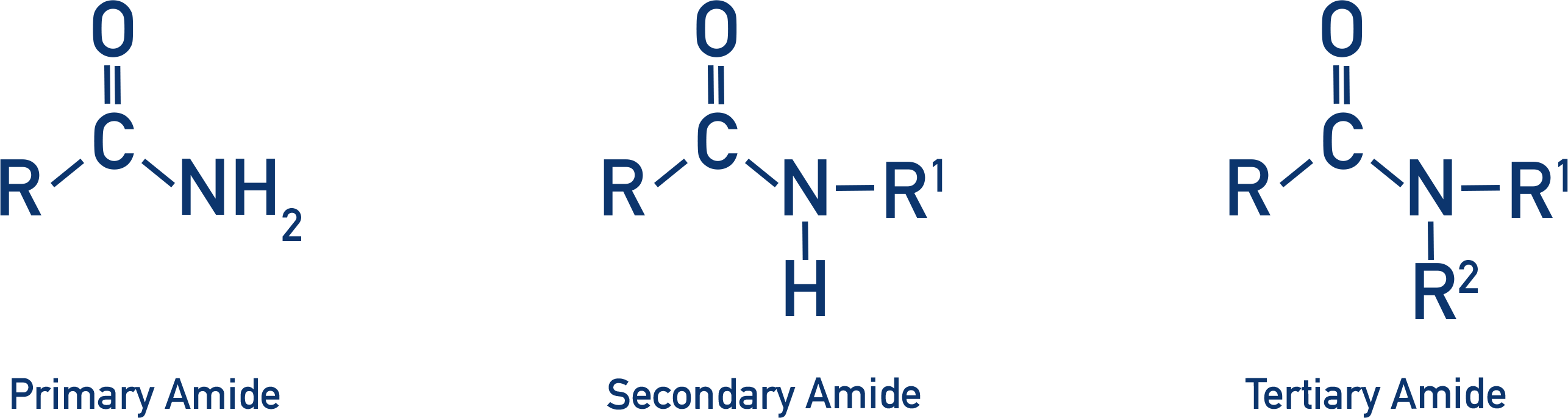
amides
nitrogen with a lone pair of electrons bonded to a carbon that is ALSO bonded to a carbonyl group (=O). can be primary, secondary, or tertiary
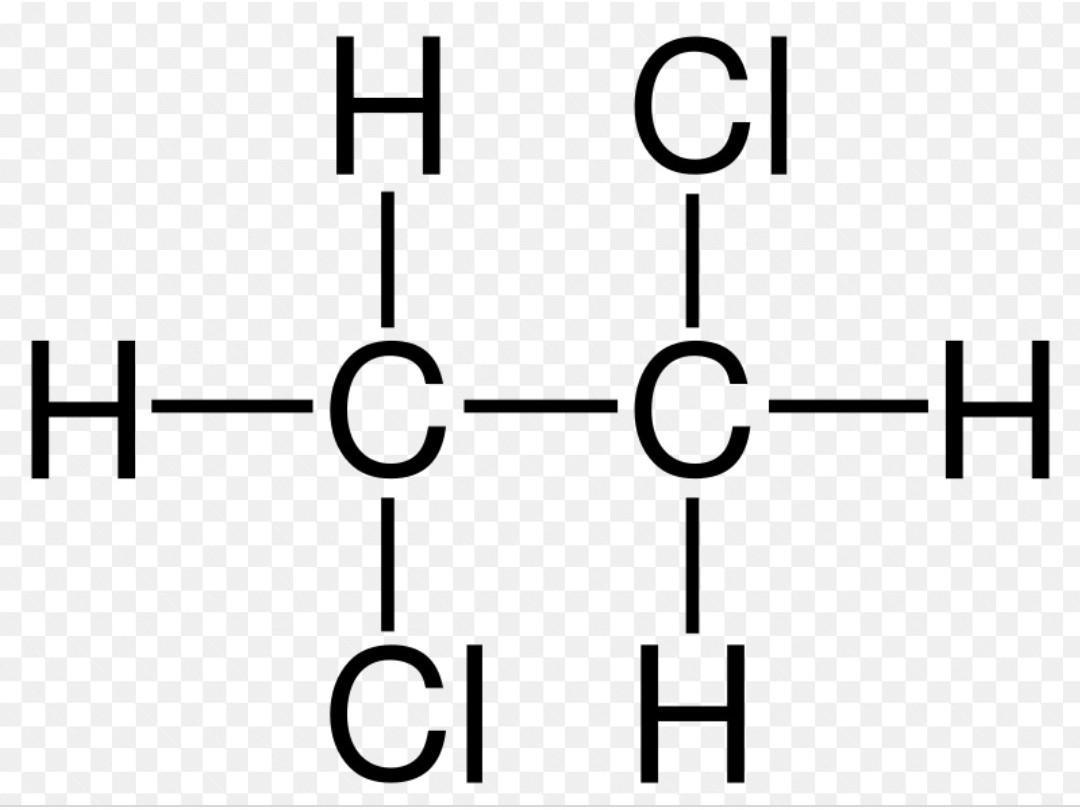
haloalkanes
alkanes which contain at least 1 HALOGEN in place of a hydrogen, e.g. 1,2-dichloroethane
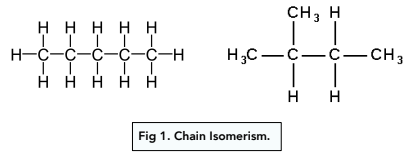
chain isomers
compounds have the same molecular formula but differ in the arrangement of their carbon skeletons.
position isomers
the same functional groups are present but in different locations on the carbon chain

functional group isomers
have the same molecular formula but different functional groups
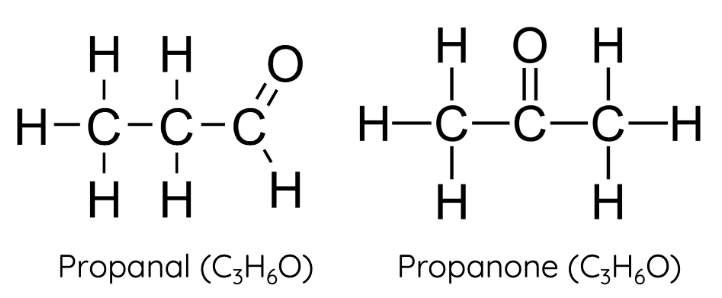
homologous series
compounds with the same functional group
properties of alkanes
chemical
not very reactive
this is because the carbon atoms have attained their octet of electrons through the formation of four covalent bonds
physical
colourless
odourless
melting and boiling points increase with molecular weight (due to dispersion forces)
properties of alkenes
chemical
more reactive than alkanes
because of the C=C bond, which acts as a functional group and is more reactive, as they can undergo addition reactions
can undergo addition reactions
physical
smaller members are gases, higher are liquids, containing more than 18 C are solids
generally colourless and odorless (except ethene)
insoluble in water
readily soluble in organic solvents
melting and boiling points increase with molecular weight (due to dispersion forces)
properties of alcohols
(OH) is a polar section due to high electronegativity of O
rest of chain is non-polar
therefore, larger alcohols tend to be more non-polar as the ratio of polar:non-polar region is smaller
also, solubility in water decreases with size (due to polarity)
more soluble than hydrocarbons because they can from dipole-dipole forces and hydrogen bonds (due to the presence of H attached to O)
the OH group can donate and accept hydrogen bonds to and from water molecules
isomers with shorter carbon chains tend to be more soluble because of repulsion between their non-polar regions and water is minimised
e.g. 2-propanol is more soluble in water than 1-propanol AND methyl-2-propanol is more soluble than 1-butanol
higher boiling and melting points than hydrocarbons with the same number of carbon atoms because the OH group allows the formation of dipole-dipole forces and H bonds
larger alcohols have higher M&B ponts (increased bc of the dispersion forces)
straight-chained alcohols are able to form greater dispersion forces due to greater surface area between molecules
therefore, straight-chains usually have higher melting and boiling points than other isomers
safe handling of organic substances
use of PPE
safety goggles
closed shoes
lab coats
gloves
many substances are flammable, so avoiding sources of ignition (use something like a hot water bath instead if heat is required)
use a fume-hood for toxic substances, as well as volatile substances
store away from oxidising agents, as they are easily oxidised
acknowledge the MSDS
environmental implications of obtaining and using hydrocarbons
overall negative
hydrocarbons have been increasingly used in the last 250 years as a source of energy via combustion
high concentrations of CO2 in the atmosphere are now linked to this combustion
global warming is largely due to the mass combustion of hydrocarbons by humans for the last 200 years
extraction of crude oil is environmentally damaging
heavy industry that is now possible due to the reliable source of energy from hydrocarbon fuels is also a heavy polluter of the environment
economic implications of obtaining and using hydrocarbons
overall positive
reliable source of energy
long-haul transportation industries have boomed, including global travel and aviation
the development of the plastics industry which lead to the computing and I.T. age
development and advanced drugs and pharmaceuticals for improved quality of life
employment booms, e.g. mass CD production
sociocultural implications of obtaining and using hydrocarbons
positives
development of advanced drugs and pharmaceuticals for improved quality of life
efficient and cheap form of heating households
town electricity supplies possible
extended work hours and increased productivity
negatives
exposure to hydrocarbons and health issues
increasing cost associated with overfull landfill and inefficient recycling practices
normalisation of energy-demanding lifestyles are hard to repeal
extended work hours and increased productivity
safe disposal of organic substances
should not be poured down the drain as they can be toxic, flammable, easily oxidised, carcinogenic, etc
small quantities can be left in shallow vessels in the fume hood and be left to evaporate
larger quantities should be disposed of in an appropriate and designated waste container, which should be located in the fume hood
these containers are collected by waste disposal personnel
halogenated hydrocarbons are required to be disposed in ‘halogenated organic waste’ container because they are toxic upon inhalation and ingestion— includes any compounds that contain F, Cl, Br, and I