STRUCTURAL EFFECTS ON ACIDITY AND BASICITY
1/98
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
99 Terms
ACID
pH<7
ACID
Sour taste
ACID
Turns litmus paper to red
ACID
React with bases to
neutralize their properties
ACID AND BASE
Conducts electricity
BASE
pH>7
BASE
Bitter taste
BASE
Turns litmus paper to blue
BASE
React with acids to
neutralize their properties
Arrhenius Acids and Bases
concept is based on whether the substance yields H+ or OHin aqueous solutions.
acid
is a substance that, when dissolved in water, increases the concentration of
H+
ions.
base
is a substance that, when dissolved in water, increases the concentration of
OH- ions.
Arrhenius Acids and Bases Limitations
restricted to aqueous solutions
Arrhenius Acids and Bases
defined based on production of ions
Arrhenius Acids and Bases
it can’t exist when not dissolved in water
higher
Acid H+ ions have _ aq solns
Brønsted-Lowry Acids and Bases
concept is based on the fact that acid–base reactions involve the transfer of H+
ions/proton from one substance to another.
acid
is a substance (molecule or ion) that donates a proton to another substance;
proton donor.
base
is a substance that accepts a proton; proton acceptor.
proton
The transfer of a _ always involves both an acid (donor) and a base (acceptor).
Brønsted-Lowry Acids and Bases
Not limited to aqueous solutions.
Brønsted-Lowry
diff people and did not know eo but they discovered the same thing
Brønsted-Lowry Acids and Bases
can be in a gaseous form
# of electrons
H atom and H ions difference
mass #
number of protons and neutrons
base
acid cannot exist without
Conjugate Acid-Base Pairs
In any acid–base equilibrium, both the forward reaction (to the right) and the reverse
reaction (to the left) involve proton transfer.
conjugate base
Every acid has a _, formed by removing a proton from the acid.
◦ HA – A-
conjugate acid
Every base has a _, formed by adding a proton to the base.
◦ H2O – H3O+
NO2- (aq)
HNO2 acid to cb
H3O+ (aq)
H2O (l) base to ca
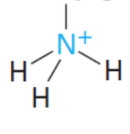
NH4+ (aq)
NH3 (aq) base to ca
OH- (aq)
H2O (l) acid to cb
H2O
may either be a proton acceptor or proton donor depending on the reaction (depends on its partner)
acid
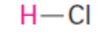
base
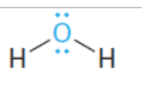
conjugate acid
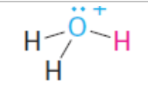
conjugate base

base
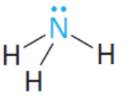
cb
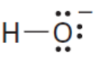
ca
acid
Any species that has a hydrogen can potentially act as an _.
base
Any species that has a lone pair can potentially act as a _.
amphiprotic
A substance capable of acting as either an acid or a base is called _.
amphiprotic
An _ substance acts as a base when combined with something more strongly
acidic than itself and as an acid when combined with something more strongly basic
than itself.
Acidity
_ is a measure of the tendency of a compound to lose a proton, whereas basicity
is a measure of a compound’s affinity for a proton.
basicity
Acidity is a measure of the tendency of a compound to lose a proton, whereas _
is a measure of a compound’s affinity for a proton.
better or stronger
acid base ca cb depends on which is the _ acid or base depending on the rxn
acid
A strong _ has a strong tendency to lose a proton; thus, its conjugate base is weak
because it has little affinity for the proton.
conjugate base
A strong acid has a strong tendency to lose a proton; thus, its _ is weak because it has little affinity for the proton.
weak acid
A _ has little tendency to lose a proton; thus, its conjugate base is strong
because it has a high affinity for the proton.
conjugate base
A weak acid has little tendency to lose a proton; thus, its _ is strong because it has a high affinity for the proton.
stronger
The _ the acid, the weaker its conjugate base.
weaker
The stronger the acid, the _ its conjugate base.
ClO4-, HS-, PH3, CO32-
what is the cb of HClO4, H2S, PH4+, HCO3-?
HCN, HSO4-, H3O+, H2CO3
what is the ca of CN-, SO42-, H2O, HCO3-?
H2SO3, HF, HPO42-, HCO+
write the formula for the ca of each of the following:
HSO3-, F-, PO43-, CO
Ka and pKa
Acid and base strength are expressed in _ 2
HCl, HBr, HI, HNO3,
HClO3, and HClO4)
The most common strong acids include six monoprotic acids (_), and one diprotic acid (H2SO4).
H2SO4
◦ The most common strong acids include six monoprotic acids (HCl, HBr, HI, HNO3,
HClO3, and HClO4), and one diprotic acid (_).
strong acids
The most common _ include six monoprotic acids (HCl, HBr, HI, HNO3,
HClO3, and HClO4), and one diprotic acid (H2SO4).
HCl, HBr, HI, HNO3,
HClO3, and HClO4, and H2SO4
most common strong acids (7)
Strong bases and Strong acids
_ 2 ionize completely in water.
strong bases
The most common _ include hydroxides of the alkali metals (NaOH, KOH) and hydroxides of alkaline earth metals (Ca(OH)2, Sr(OH)2, and Ba(OH)2).
NaOH, KOH
The most common strong bases include hydroxides of the alkali metals (_ 2) and hydroxides of alkaline earth metals (Ca(OH)2, Sr(OH)2, and Ba(OH)2).
Ca(OH)2, Sr(OH)2, and Ba(OH)2
The most common strong bases include hydroxides of the alkali metals (NaOH, KOH) and hydroxides of alkaline earth metals (_ 3).
NaOH, KOH, and Ca(OH)2, Sr(OH)2, and Ba(OH)2
most common strong bases 5
Weak acids & Weak bases
_ 2 ionize partially in water.
strong acids
→
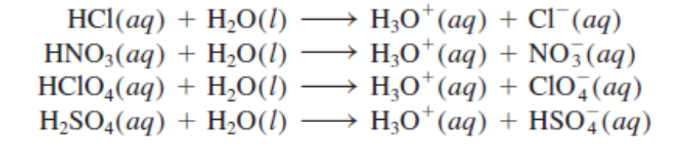
strong bases
→
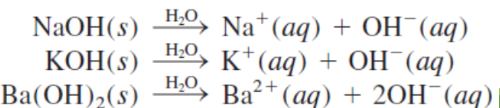
weak acids
←→
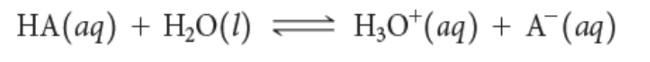
weak bases
←→
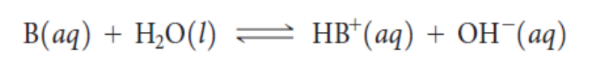
Ka
(acid-dissociation constant)
The higher the Ka, the stronger the acid.
Ka
(acid-dissociation constant) for an acid HA is:
Kb
(base-dissociation constant)
The higher the Kb, the stronger the base.
Kb
(base-dissociation constant) for a base B is:
higher; stronger
The _ the Ka, the - the acid.
The _ the Kb, the - the base.
Ka formula
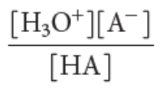
Kb formula
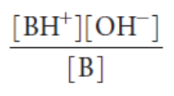
larger pKa
A stronger acid (larger Ka) has a smaller pKa, and a weaker acid (smaller Ka) has a _.
weaker acid (smaller Ka)
A stronger acid (larger Ka) has a smaller pKa, and a _ has a
larger pKa.
smaller pKa
A stronger acid (larger Ka) has a _, and a weaker acid (smaller Ka) has a
larger pKa.
stronger acid (larger Ka)
A _ has a smaller pKa, and a weaker acid (smaller Ka) has a
larger pKa.
pKa formula
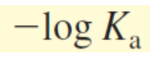
very strong acids
pka <1
moderately strong acids
pKa=1-3
weak acids
pKa=3-5
very weak acids
pKa=5-15
extremely weak acids
pKa>15
weaker acid to stronger acid
CH3CH2OH to HCl
weaker base to stronger base
HCl to CH3CH2OH
smaller pKa
stronger acid (larger Ka) has a _
larger pKa
weaker acid (smaller Ka) has a_
1.0 x 10-14 at 25°C
(Ka)(Kb) = Kw =
14 at 25°C
pKa + pKb =
.
Ka increases Kb decreases and vice versa
pKa increases pKb decreases and vice versa
divide 1×10-14 by the Ka then u get Kb
Ka derive Kb
negative logarithm of ka
derive pKa from Ka
14-pka
derive pkb from pka