Unit 3 Honors Chemistry
1/43
Earn XP
Description and Tags
Guaranteed 100%
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
44 Terms
When dealing with the loss of electrons from transition metals, you always lose the electrons from the [...] subshell before you remove from the [...] subshell. This is because the ones in the [...] subshell are in a higher shell and are thus your [...] electrons
s; d; s; valence
When matter like glass allows light to pass through we call it?
Transmission
What is the formula to calculate the wavelength or frequency?
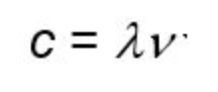
Which electromagnetic wave has the most energy?
Gamma
[...] is when all the electrons in a subshell are not spin paired
Paramagnetic
[...] state means an electron has jumped from one energy level to another and the electron configuration is thus out of sequence
Excited
What does the color we see when light hit an object depend on?
Which shell the electron moves from
Metals that are [...] are generally attracted by a magnet
paramagnetic
When writing the electron congiuration, we always fills the subshells from [...] energy to [...] energy
lowest; highest
What is the formula to calculate the energy of a wave with a given frequency?
E=hv
What is the formula to determine how many electrons a shell can hold?
2n2
What are the 3 times zeroes are signifcant?
1. When they are between two integers taht are significant.
2. When they they follow a number and a decimal
3. When a number (1-9) is before the decimal,all the zeroes shown are significant.
Continous spectrums are produced by hot objects like the sun, volcanic lava and incandescent lamps which produce all the colors of the rainbow (ROY G BV) while [...] occurs when an electron jumps to a higher energy level as a result of absorbing energy
emission spectrum
The amount of energy that you need to excite an electron based on the photoelectric is called
Threshold frequency
Which type of emission is created by gaseous atoms and creates a specific pattern of colors called a line spectrum?
Spectral Emission
[...] are produced by hot objects like the sun, volcanic lava and incandescent lamps which produce all the colors of the rainbow (ROY G BV) while emission spectrum occurs when an electron jumps to a higher energy level as a result of absorbing energy
Continuous spectrums
Light striking the surface of an object and causing an electron to be ejected is called?
photoelectric effect
What is the value for the speed of light
3.0 x 108
What do the 4 quantum numbers represent?
Shell, subshell, orbital and spin
The units for wavelength are usually given as nanometers but you need to change them to meters when determining the frequency by multiplying the value meter value given by [...]?
1.0 x 10-9
When Matter can absorb light that results in the increase of its temperature (conversion of radiative energy into thermal energy) we call it?
absorption
What is the value for h?
6.626 x 10-34
Each orbital can hold a maximum of [...] electrons
2
When multiplying two values in scientific notation, you [...] the coefficients and [...] the exponents
multiply; add
Which electromagentic wave has the least energy?
Radio frequency
As the frequency increases, the wavelength [...] and the energy [...]
decreases; increases
Which rules says that no two electrons in the same atom can have identical sets of quantum numbers.
Pauli exclusion principal
The units for wavelength are usually given as [...] but you need to change them to [...] when determining the frequency by multiplying the value meter value given by 1.0 x 10-9?
nanometers; meters
What are the colors of visible light?
ROYGBV
The s subshell has 1 orbital, the p subshell has 3 orbitals, the d subshell has [...] orbitals and the f subshell has 7 orbitals
5
[...] is when all the electrons in a subshell are spin paired.
Diamagnetic
The s subshell has 1 orbital, the p subshell has 3 orbitals, the d subshell has 5 orbitals and the f subshell has [...] orbitals
7
The key to scientific notation is that you can only have [...] to left of decimal point
one number
When filling the orbitals in a subshell, all the orbitals must first be occupied by a [...] spin before a [...] spin can be placed according to [...]
positive; negative; Hunds rule
In which type of emission does a solid object emits light when heated
Black Body Emission
The s subshell has 1 orbital, the p subshell has [...] orbitals, the d subshell has 5 orbitals and the f subshell has 7 orbitals
3
Which color is most energetic and which is the least?
Violet is the most and red is the least
When wiriting a value less than 1 into scientific notation, you need to move the decimal point to the [...]
right
The s subshell has [...] orbital, the p subshell has 3 orbitals, the d subshell has 5 orbitals and the f subshell has 7 orbitals
1
When writing a number with a value of 10 or larger into scientific notation, you need to move the decimal point to the [...]
left
When Photons bounce off the surface of some matter, like a mirror we call it
reflection
When dividing two values in scientific notation, you [...] the coefficients and subtact the exponents
divide
[...] state configuration means you are filling the electrons in order from lowest energy to highest energy
Ground
When dividing two values in scientific notation, you divide the coefficients and [...] the exponents
subtract