chapter 11 - modern atomic theory
1/60
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
61 Terms
rutherford’s atoms
positive core with negatively charged electrons moving about it. nucleus is very small compared to atom so electrons make up rest of an atom
max Planck
discovered that atoms and molecules emit energy only in whole number multiples of certain well defined quantities
quanta
energy can be released only in certain definite amounts
plancks quantum theory/radiation
whcih is the emission and transmission through space of energy in the form of wave
wave
vibrating disturbance by which energy is transmitted
wavelength
is the distance between identical points on successive waves, usually expressed in units of m, cm, or nm
frequency
number of waves that pass through a particular point in one second, usually measured in units of hertz (Hz) (1Hz = S^-1)
amplitude
is the vertical distance from the midline of a wave to the peak or through
speed of a wave (u)
is given by the product of its wavelength and its frequency
photons
one can think of a beam of light traveling through space as a steam of tiny packets of energy
longer the wavelength of light
lower the energy
shorter the wavelength
higher the energy
common electromagnetic waves features
speed at which they travel, 3.00 × 10^-18 meters per second (m/s) or 186,000 miles per second, electromagnetic waves is c = frequency/wave length v
what does C equal
2.998 × 10^8 m/s
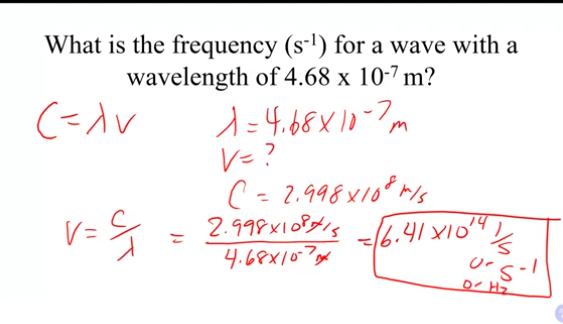
what is the frequency (s^-1) for a wave with a wavelength of 4.68 × 10^-7 m?
2.998×10^8/4.68 × 10^-7 = 6.41 × 10^14
what is the wavelenght in nannometer for a wave with frequency of 5.6 × 10^14 Hz
(2.998×10^8/5.6×10^14) = 5.35 × 10^-7 m (1nm/10^-9) = 535 nm

quantum
smallest quantity of energy that can be emitted in the form of electromagnetic radiation
energy equation
E = hv
h plancks constant
6.63 × 10^-34
what is the energy in J associated with a wave that has a wavelength of 465nm?
465nm(10^-9m/1nm)=4.65×10^-7m
(6.63×10^-34J)(2.998×10^8)/4.65×10^-7m = 4.27×10^-19
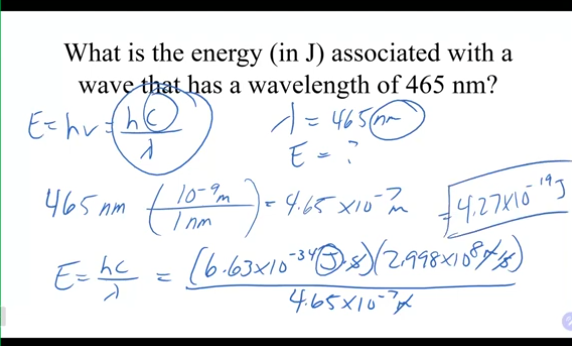
what is the energy (in J) associated with a wave that has a frequency of 2.87 × 10^14 s-1
E=hv
(6.63 × 10^-24J)(2.87×10^14 S^-1) = 1.90 × 10^-19 J

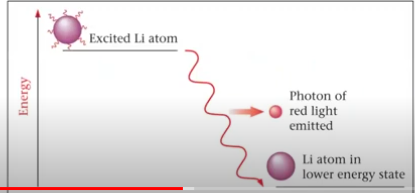
graph of an excited lithium atom emitting a photon of red light to drop a lower energy state
emission spectra
of various substances is either continuous or line spectra of radiation emitted by the substances
the emission of a substance is obtained
by energizing a sample or material either with thermal energy or with some other energy (like, high-voltage electrical discharge if substance is gaseous)
line spectra
radiation is identified by the appearance of bright lines in the spectra
niels Bohr theory
the idea of electrons moving in circular orbits
restriction: single electron in hydrogen atom could be located only in certain orbit
are quantized
principal quantum number
n, ground state/level
excited state or excited level
the stability of the electron diminishes for n = 2, 3 …
de Broglie
if light rays could behave like a stream of particles (photons), then perhaps particles like electrons can posses wave properties
an electron bound to a nucleus behaves like a standing wave
Heisenberg (BREAKING BAD REFERENCE!!!!)
impossible
electron density
gives the probability that an electron will be found at a particular region in an atom
orbital or atomic orbital
a 1s orbital is merely a sphere, all s orbitals are spherical in shape but different in size
although
p orbitals
quantum umber #2, there are three 2p orbitals
2px, 2py, 2pz
the letter subscript indicate the axes along which the orbitals are oriented
d orbital
n = 3 we have 5 3d orbitals
3dxy, 3dyz, 3dxz, 3dxz-y2, and 3dz2
4f orbitals in terms of their boundary surfaces
the f orbitals are a set of 7 degenerate orbitals
the set can hold up to 14 electrons
f orbitals available: 4f, 5f, 6f, 7f
electron configuration
tells us how electrons are distributed among the various atomic orbitals
Hydrogen, z = 1 example
1s^1
pauli exclusion principle
no two electrons in an atom can have the same four quantum numbers
arrows have to go up and down for the electron configuration
helium, z = 2
1s²
lithium, z = 3
1s²2s^1
Beryllium, Z = 4
1s²2s²
Boron, z = 5
1s²2s²2p^1
hund’s rule
most stable arrangement of electrons in subshells is the one with the greatest number of parallel spins
Carbon, z = 6
1s²2s²2p²
Nitrogen, z = 7
1s²2s²2p³
oxygen, z = 8
1s²2s²2p^4
fluorine, z = 9
1s²2s²2p^5
Neon, z = 10
1s²2s²2p^6
argon, z = 18
1s²2s²2p^6 3s²3p^6
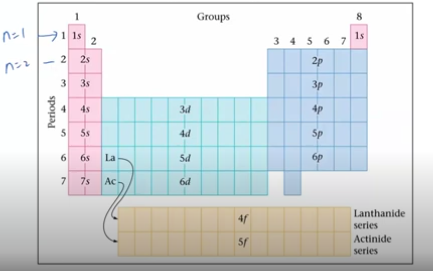
aufbau principle
proton are added one by one to the nucleus to build up the elements, electrons are similarly added to the atomic orbitals
K, z = 19
1s²2s²2p^6 3s²3p^6 4s^1
transition metals
either incompletely filled d subshells or readily give rise to cations that have incompletely filled d subshells
chromium and copper are exceptions
4s^1 3d^5
4s^1 3d^10
lanthanide series
incompletely filled 4f subshells or readily give rise to cations that have incompletely filled 4f subshells
the orbitals being filled for elements in various parts of the periodic table
Na and Na+
Na = 11, 1s²2s²2p^6 3s^1
Na+ = 10, 1s²2p²2p^6
N and N³-
N = 7, 1s²2s²2p³
N³- = 10, 1s²2s²2p^6
Fe, Fe²+ and Fe³+
Fe = 26 1s²2s²2p^6 3s² 3p^6 4s² 3d^6
Fe²+ = 24 = 1s²2s²2p^6 3s² 3p^6 3d^6
Fe³+ = 23 = 1s²2s²2p^6 3s² 3p^6 3d^5
(get the S first for transition metals)
Ca²+, Br-, Mn^4+, Ni²+
Ca = 18, 1s²2s²2p^6 3s²3p^6
Br = 36, 1s²2s²2p^6 3s²3p^6 4s² 3d^10 4p^6
Mn = 21, 1s²2s²2p^6 3s²3p^6 3d³
Ni = 5, 1s²2s²2p^6 3s²3p^6 3d^8
valence electrons
electrons in the outermost (highest) principle energy level of a an atom
ionization energy
energy required to remove an electron from an individual atom in the gas phase
M(g) → M^+ + e^-
electron affinity
energy change associated with the addition of an electron to a gaseous atom