chapter 1 principles of biochemistry
1/108
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
109 Terms
what is biochemistry
explains biological processes at the molecular and cellular level
relies heavily on the quantitative analysis of data
often studies in vitro (outside a living cell) systems
fermentation
the conversion of rotting fruit or grain into alcohol solutions through the action of yeast (yeast enzymes act as a catalyst)
what type of process is alcohol fermentation?
anaerobic
Buchner’s experiment
Buchner showed that carbon dioxide and ethanol were produced in vitro from sugar using brewer’s yeast in 1897
credited with proposing that enzymes helped speed up the reaction
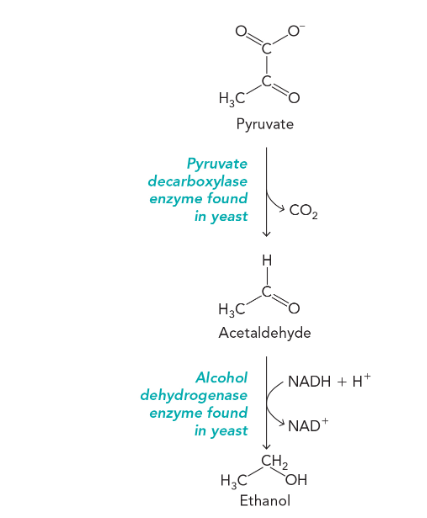
alcoholic fermentation reaction
pyruvate decarboxylase converts pyruvate into acetaldehyde and carbon dioxide
alcohol dehydrogenase reduces acetaldehyde to ethanol
the second part involving alcohol dehydrogenase is a redox reaction
catalysts like proteins or RNA are
biomolecules that increase the rate of biochemical reactions by lowering the activation energy the reactants need
catalysts are found in
all living cells
catalysts are responsible for which reactions?
aerobic respiration
fermentation (anaerobic)
nitrogen metabolism
energy conversion
programmed cell death
applied biochemistry is used in the following fields
environmental science
biotechnology
agriculture
pharmaceuticals
clinical diagnostics
commercial products
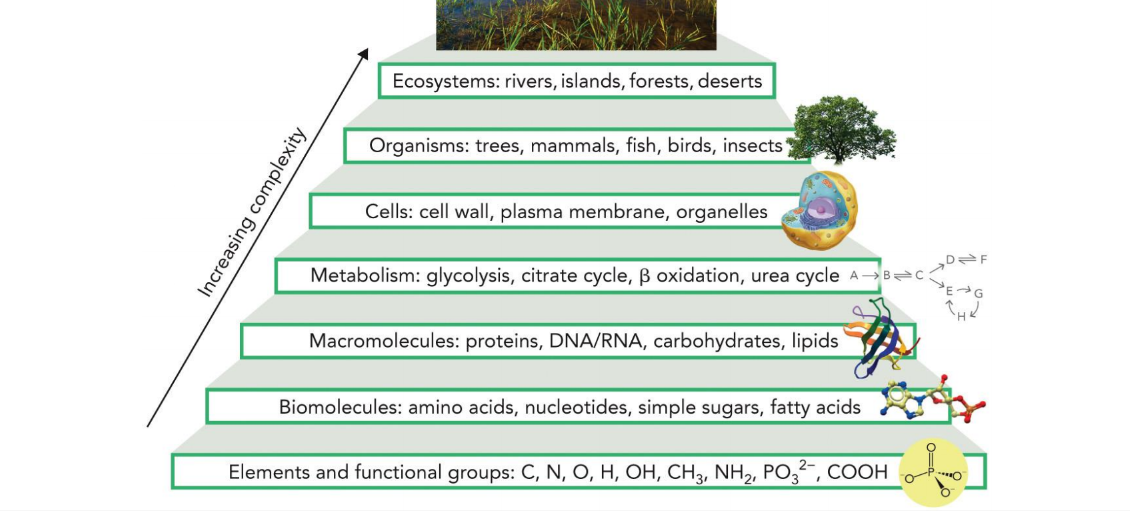
organizational hierarchy of biochemistry
elements and functional groups → biomolecules → macromolecules → metabolism → cells → organisms → ecosystems
trace elements are used as what in proteins?
cofactors
cofactors
non-protein chemical compounds that are required for an enzyme’s role as a catalyst
coenzymes
cofactors derived from vitamins and nutrients
trace elements
manganese, iron, cobalt, copper, zinc
essential ions that play a role in cell signaling
calcium, chloride, magnesium, potassium, sodium
97% of the weight of most organisms consists of
C, H, O, N, P, S
six most abundant functional groups in biomolecules
amino, hydroxyl, sulfhydryl, phosphoryl, methyl
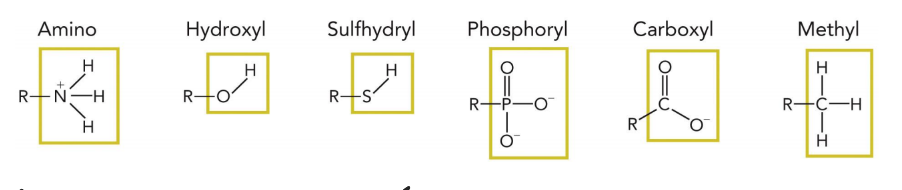
which functional group only has one protonation state?
methyl
four major types of biomolecules
amino acids, nucleotides, simple sugars (mono and disaccharides), fatty acids
amino acids primary cellular functions
protein function
neurotransmission
nitrogen metabolism
energy conversion
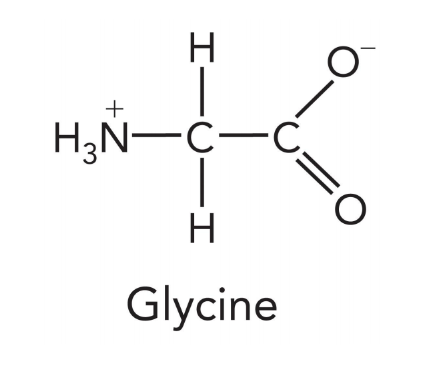
amino acids are
nitrogen containing molecules that are the building blocks of proteins
the bond that links amino acid chains
peptide bonds
how do amino acids differ from each other?
the side chain
anything over 60 amino acids is a ?
protein
nucleotides primary cellular function
nucleic acid function
energy conversion
signal transduction
enzyme catalysis
nucleotides consist of
nitrogenous base
pentose sugar
1-3 phosphate groups
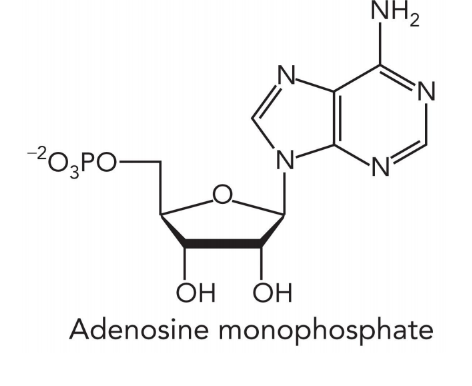
nucleosides consist of
nitrogenous base
pentose sugar
examples of nucleotides
ATP
cAMP
NAD+
simple sugar primary cellular functions
energy conversion
cell wall structure
cell recognition
nucleotide structure
simple sugars are
monosaccharides and disaccharides
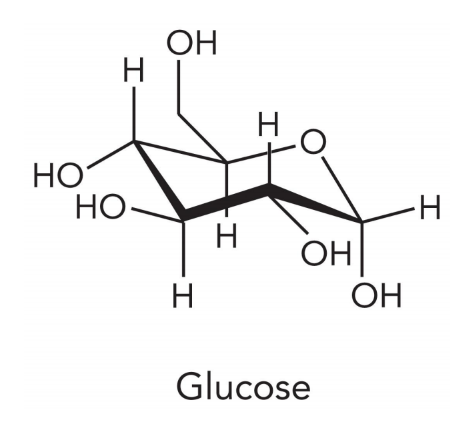
carbohydrates contain only
C, H, and O atoms
in carbohydrates the hydrogen to oxygen ratio is
2:1
fatty acids primary cellular functions
cell membranes
energy conversion
cell signaling
energy storage
fatty acids are
amphipathic molecules
amphipathic molecules
hydrophobic and hydrophilic chemical properties in one molecule
fatty acids consist of
carboxyl group
hydrocarbon chain
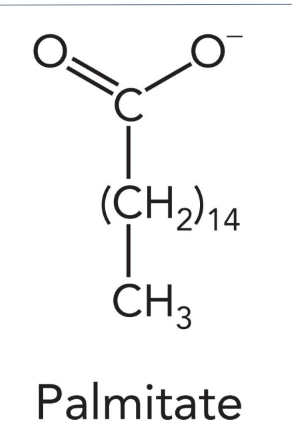
saturated fatty acid
solid at room temp, no double bonds (animal fats)
unsaturated fatty acid
liquid at room temp, double bonds (oils)
most abundant macromolecules
proteins
nucleic acids (RNA/ DNA)
polysaccharides (starch, cellulose, amylose, glycogen)
macromolecules are
chemical polymers of biomolecules
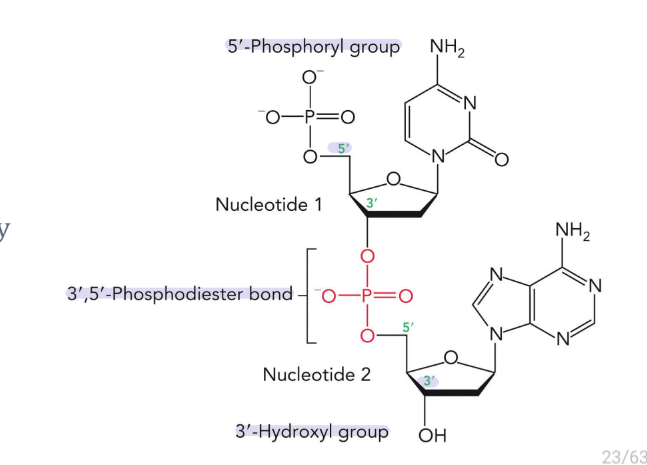
nucleic acids are covalently linked by
3’, 5’-Phosphodiester bond
proteins always start from which terminal and ends at which terminal?
amino to carboxyl
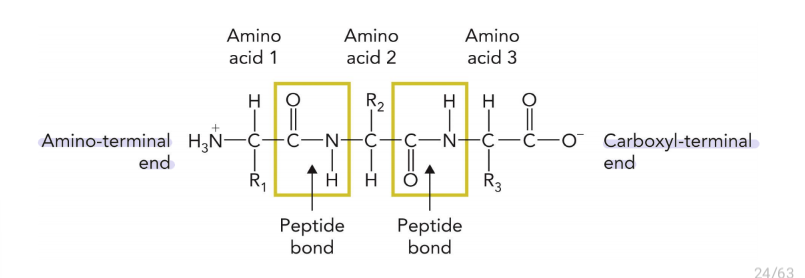
the chemical properties of proteins are determined by
different side chains in the amino acid
polysaccharides are
mixtures of different simple sugars or just repeating glucose molecules linked by glycosidic bonds
glycosidic bonds are either
α(1→4)
β(1→4)
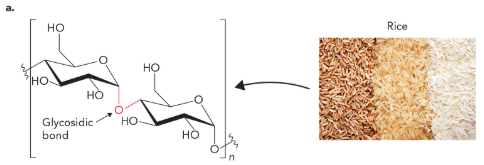
amylose (starch, glucose polymer) contains a
α(1→4) glycosidic bond
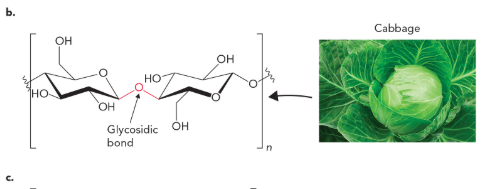
cellulose (glucose polymer) contains a
β(1→4) glycosidic bond
chitin contains a β(1→4) glycosidic bond linking
N-acetylglucosamine units
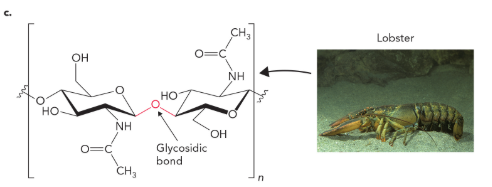
which enzyme do we lack, and which bond can humans not hydrolyze
we lack cellulase needed to hydrolyze β(1→4) glycosidic bonds
metabolite
small biomolecules that serve as reactants and products in biochemical reactions within cells
metabolic pathways
enable cells to coordinate and control complex biochemical processes in response to available energy
examples of metabolic pathways
glycolysis and gluconeogenesis (glucose metabolism)
citrate cycle (energy conversion)
fatty acid oxidation and biosynthesis (fatty acid metabolism)
metabolic flux
the rate at which reactants and products are interconverted in a metabolic pathway
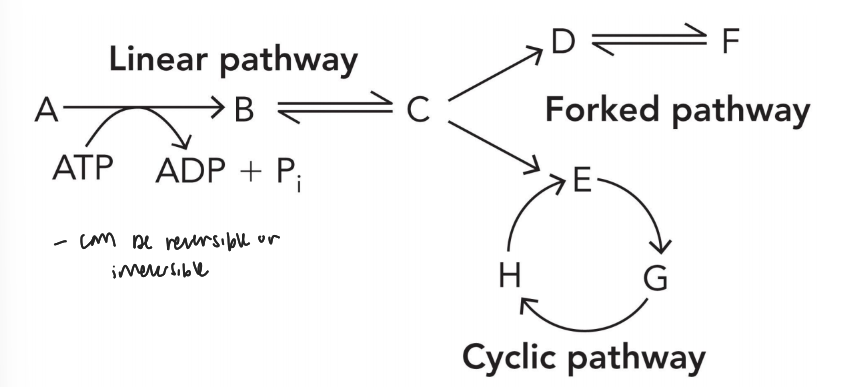
metabolic pathway formats
linear (generates a single product used for next reaction)
forked (generates two products that undergo separate reaction)
cyclic (generates several products that regenerate during each turn of the cycle)
anabolic pathway
assembling macromolecules from monomers, requires energy
catabolic pathway
disassembling macromolecules into monomers, releases energy
form of energy needed to assemble many macromolecules
ATP
when ATP levels in the cell are high
anabolic pathways are favored
when ATP levels in the cell are low
catabolic pathways are favored
the flux of metabolites through a pathway depends on
activity of the enzyme in the pathway
intracellular concentrations of reactants and products (le chatelier’s principle)
reactions in metabolic pathways can be either
reversible or irreversible
living cells are highly what and surrounded by what?
ordered structures surrounded by a lipid membrane
in order to support metabolic processes cells either
obtain energy from the sun or from oxidation-reduction reactions
nucleolus
site of ribosome assembly
ribosomes
RNA-protein complexes that mediate protein synthesis
mitochondria
responsible for many of the metabolic reactions involved in energy conversion and production of ATP
peroxisomes
containing enzymes for forming or destroying peroxides
lysosomes
involved in the degradation and detoxification of macromolecules
endoplasmic reticulum
highly invaginated membrane structures that sequester ribosomes for protein synthesis
golgi apparatus
membranous structure involved in protein translocation within the cell and facilitating protein secretion at the plasma membrane
individual cells communicate with one another in response to environmental changes using
signal transduction
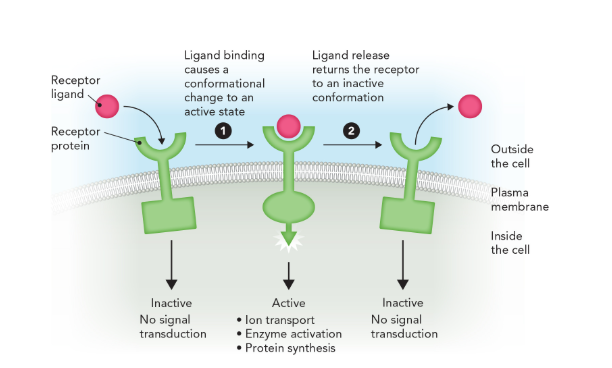
ligand
a small molecule that is often a metabolite, hormone, or peptide and which binds to target proteins (receptors) and alters their structure and function to control biochemical processes
transmembrane receptor proteins are able to transduce extracellular signals across the membrane upon ligand binding through
changes in shape (conformational changes) which activates the receptor and affects intracellular activity (increases enzyme activity)
the circulatory system allow
biomolecules to travel throughout an organism
transports signaling molecules
distributes metabolic fuel (carbohydrates, and lipids)
primary tissues and organs involved in controlling metabolic function in humans
brain (nerve center)
liver (metabolic control center)
skeletal muscle (mechanical work and glucose homeostasis)
intestines (nutrient absorption)
adipose tissue (energy storage and hormonal signaling)
kidneys (water, nitrogen, and electrolyte balance)
heart (pumps blood throughout the circulatory system)
lungs (exchange oxygen and carbon dioxide gases with the atmosphere to keep tissues and organs alive)
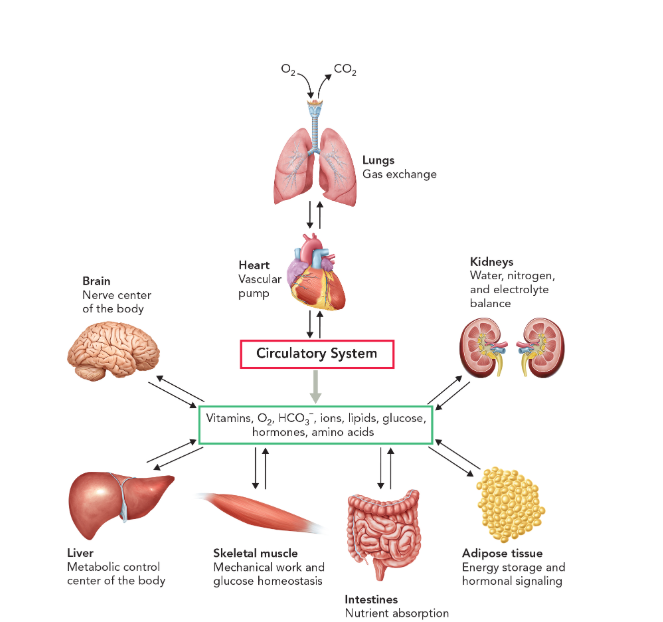
primary tissues and organs involved in controlling metabolic function in humans are coordinated by
signal transduction mechanisms
multicellular organisms depend what to distribute metabolites between specialized tissues and organs
a circulatory system
ecosystems are the
highest level of hierarchical organization
ecosystems include
cohabitation of different organisms in the same environmental niche and involve a shared use of resources and waste management
in 1952 it was reported that DNA from a bacterial virus was sufficient enough to promote
viral replication
Rosalind Franklin collected
x-ray diffraction data to determine the structure of DNA
in 1953 Watson and Crick determined that DNA is a
double helix
the DNA double helix allows
for DNA replication and for genetic information to be passed onto daughter cells
genetic information is stored in DNA as
nucleotide base pairs
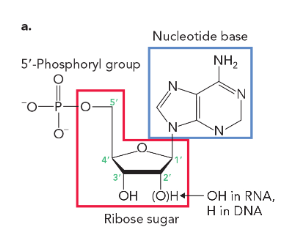
deoxyribonucleotides are monomeric units of DNA that
lack an -OH group on the C-2’ of the deoxyribose sugar (have an -H)
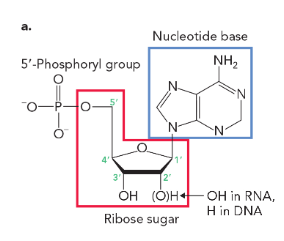
ribonucleotides are monomeric units of RNA that
contain an -OH on the C-2’ of the ribose sugar
strands of DNA are noncovalently associated through
hydrogen bonds
base pairs of DNA and RNA
in DNA (A-T, 2 hydrogen bonds), (G-C, 3 hydrogen bonds)
in RNA (A-U, 2 hydrogen bonds), (G-C, 3 hydrogen bonds)
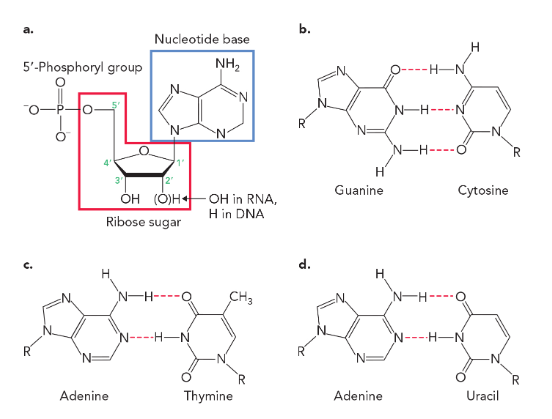
purines
adenine and guanine
pyrimidines
cytosine and thymine (uracil is a pyrimidine derivative)
how is genetic information passed down through genetic inheritance
the complementary nucleotide base pairs allows for replication of exact DNA
DNA replication is
semi-conservative
central dogma
DNA (genes/ transcription) → RNA (translation) → protein
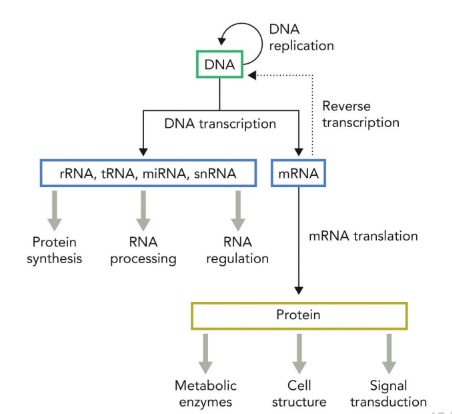
reverse transcription
conditions in which RNA molecules can be converted back to DNA (often related to virus replication)
genome
collection of genes
transcriptome
collection of DNA transcripts (RNA products) generated by DNA transcription; can refer to all possible RNA products present in an organism or cell type or to just those generated under defined conditions
proteome
collection of proteins produced by mRNA translation, either in the entire organism or under special conditions
structure determines
function
a gene is maintained through natural selection if
the DNA change leads to a beneficial change in function and structure
if a single nucleotide changes in a wild-type protein coding sequence then
the encoded protein may be functionally defective