Atomic Structure (protons, neutrons, and electrons) and Ions/Isotopes quiz 10/11
1/34
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
35 Terms
Mass, location, charge, relative mass, and actual mass: protons
mass: 1 amu
Location: inside nucleus
Charge: +1 (positive)
Relative mass: 1
Actual mass: 1.67 × 10-24g
Mass, location, charge, relative mass, and actual mass: Neutrons
mass: 1 amu
Location: inside nucleus
Charge: 0 (no charge)
Relative mass: 1
Actual mass: 1.67 × 10-24g
Mass, location, charge, relative mass, and actual mass: Electrons
mass: no mass (~ zero)
Location: outside nucleus
Charge: -1 (negative charge)
Relative mass: 1/1840
Actual mass: 9.11 × 10-28g
Amu
A relative atomic mass system that compares all other atoms to a standard atom
The standard comparison is the carbon-12 atom
1 amu = 1/12 mass of the carbon-12 atom
Contains (protons, neutrons, or electrons), charge, almost all of the ___ of the atom: nucleus
contains: protons and neutrons
Charge: positive
Almost all of the mass of the atom
Contains (protons, neutrons, or electrons), almost all of the ___ of the atom: Electron cloud
contains: electrons
Almost all of the volume of the atom
The size of an atom
incredibly small
Measured in pico meters (pm)
1 pm = 10-12m
Hydrogen (the smallest atom) measures only a 32 pm radius
How does the nucleus compare to the electron cloud in size?
very small compared to the whole atom
Density of the nucleus
10-12m
2 × 108 metric tons/ cm3
Neutral atom: atomic number and mass number
atomic number = number of protons and electrons
Mass number = sum of protons + neutrons
What determines the element and kind of atom?
Number of protons
represented by Z → Z = number of protons
Formula for calculating number of neutrons in a neutral atom
Mass # - atomic # = number of neutrons
MAN
Ion
A charged atom
List the 2 types of ions
Anion
Cation
Charge, formed when atoms ___ electrons, more ___ than ___: anion
negative ion
Formed when atoms gain electrons
More electrons than protons
Charge, formed when atoms ___ electrons, more ___ than ___: Cation
charge: positive ion
Formed when atoms lose electrons
More protons than electrons
Formula for net charge of an ion
Net charge → protons ± (reciprocal sign of the number of electrons gained or lost) = electrons
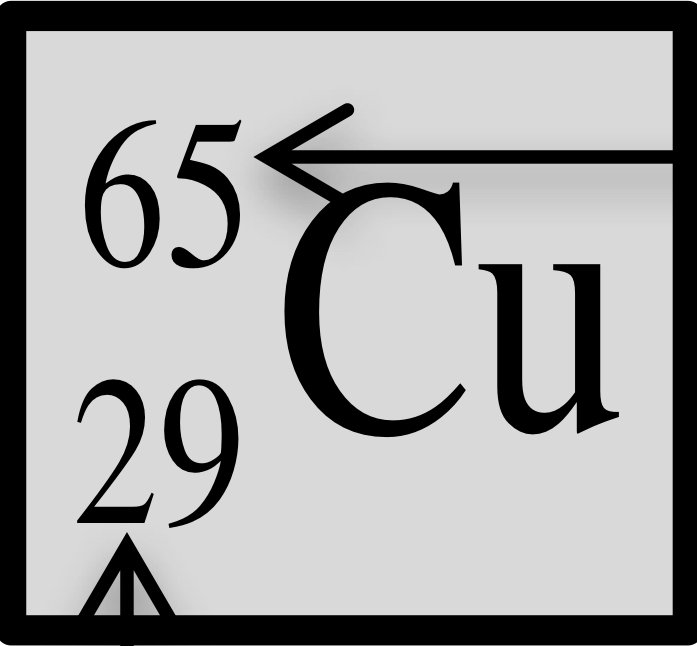
Atomic number, mass number, number of protons, neutrons, and electrons
atomic number: 29
Mass number: 65
Protons: 29
Neutrons: 36
Electrons: 29

Atomic number, mass number, number of protons, neutrons, and electrons
atomic number: 3
Mass number: 7
Protons: 3
Neutrons: 4
Electrons: 3
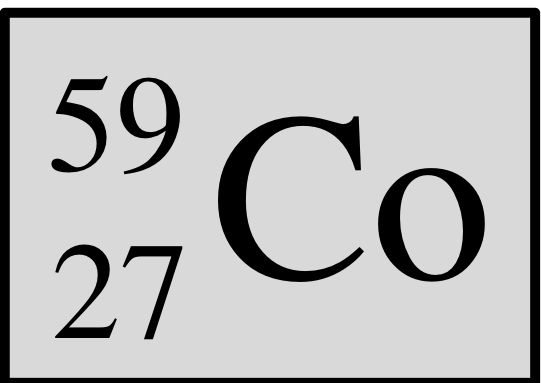
Atomic number, mass number, number of protons, neutrons, and electrons:
atomic number: 27
Mass number: 59
Protons: 27
Neutrons: 32
Electrons: 27

Atomic number, mass number, number of protons, neutrons, and electrons:
atomic number: 53
Mass number: 127
Protons: 53
Neutrons: 74
Electrons: 53
Hyphen notation
Element symbol - (mass number)
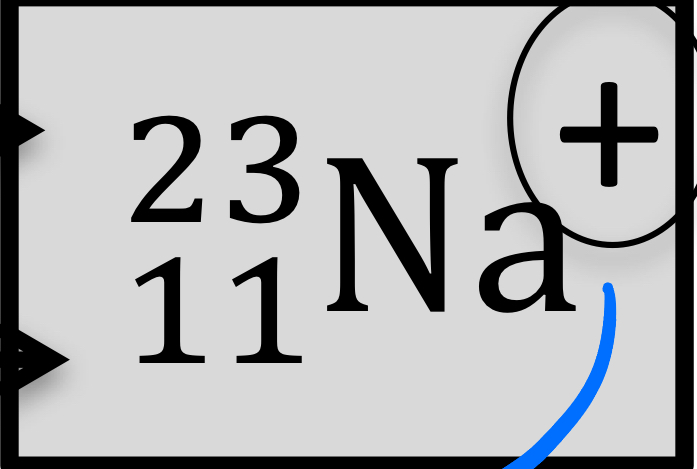
Atomic number, mass number, number of protons, neutrons, and electrons:
atomic number: 11
Mass number: 23
Protons: 11
Neutrons: 12
Electrons: 10
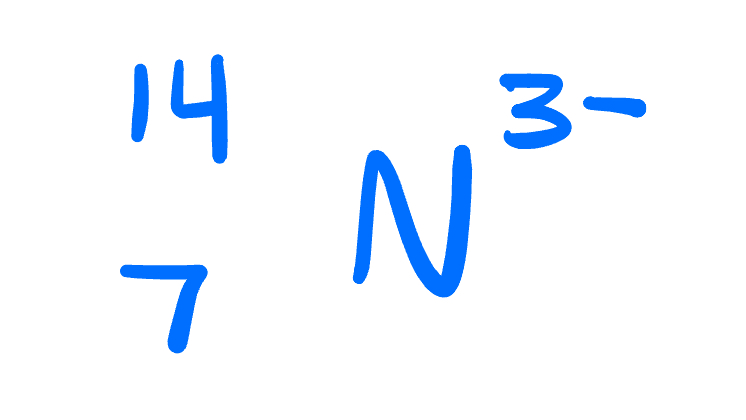
Atomic number, mass number, number of protons, neutrons, and electrons:
atomic number: 7
Mass number: 14
Protons: 7
Neutrons: 7
Electrons: 10
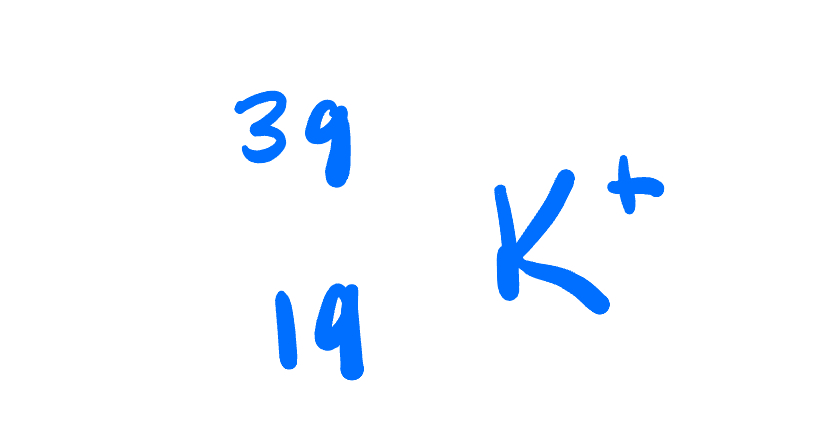
Atomic number, mass number, number of protons, neutrons, and electrons:
atomic number: 19
Mass number: 39
Protons: 19
Neutrons: 20
Electrons: 18
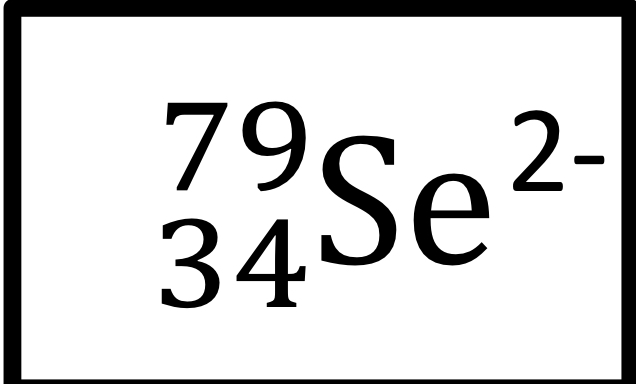
Atomic number, mass number, number of protons, neutrons, and electrons:
atomic number: 34
Mass number: 79
Protons: 34
Neutrons: 45
Electrons: 36
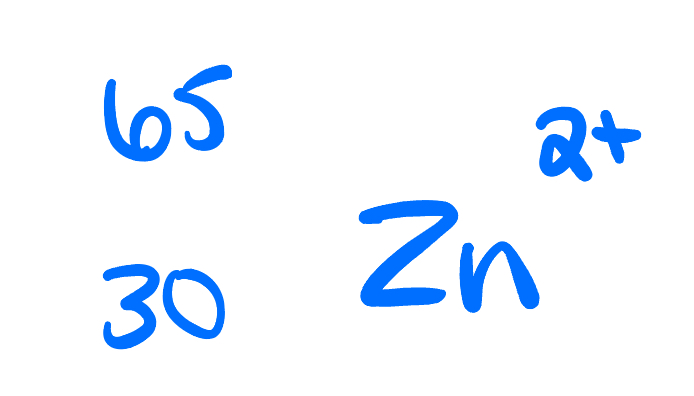
Atomic number, mass number, number of protons, neutrons, and electrons:
atomic number: 30
Mass number: 65
Protons: 30
Neutrons: 35
Electrons: 28
Isotopes
Atoms with the same number of protons (same element), but different number of neutrons
How many elements have isotopes?
Almost all of them
Are isotopes stable or radioactive?
Some isotopes are stable, and some isotopes are radioactive
Isotopes differ in number of neutrons, so they also differ in…
Mass
Since carbon has an atomic number of 6, how many protons and neutrons does carbon-12 have?
protons: 6
Neutrons: 6
Since carbon has an atomic number of 6, how many protons and neutrons does carbon-14 have?
protons: 6
Neutrons: 8
Since uranium has an atomic number of 92, how many protons and neutrons does uranium 235 have?
protons: 92
Neutrons: 143
Since uranium has an atomic number of 92, how many protons and neutrons does uranium 238 have?
protons: 92
Neutrons: 146