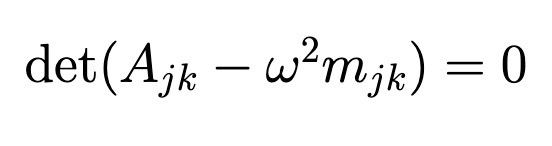Physics GRE Prep
1/128
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
129 Terms
Bohr Model, energy shell transition energy
Rydberg Formula
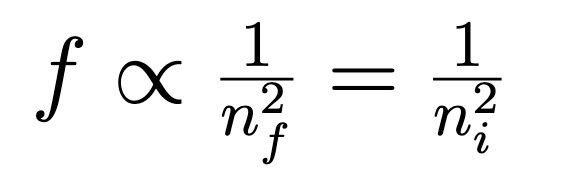
Bohr Model, angular momentum (L)
L = nh
Bohr Model, Do electrons radiate from acceleration around nucleus?
No
H Atom Pert, Relative strength of fine structure
Smaller than Bohr energies
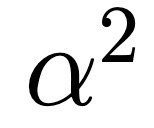
H Atom Pert, What is fine structure pertubation?
Hydrogen energy levels depend on total spin (j), m_j conserved, comes from replacing electron kinetic energy with full relativistic form and spin-orbit coupling.
H Atom Pert, Lamb Shift relative strength?
Smaller than fine structure by a factor of alpha.
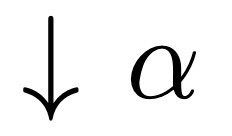
H Atom Pert, Where does Lamb Shift come from ?
Quantum electrodynamics, splits 2s and 2p energy levels with j = ½ (degenerate).
H Atom Pert, Where does Hyperfine Structure come from?
Magnetic Dipole interaction, spin-spin coupling between spins of electron and proton.
H Atom Pert, relative strength of Hyperfine Structure?
Same order of alpha as fine structure (smaller by alpha² than Bohr energies, larger by alpha than Lamb Shift). Lessened by me / mp because gyromagnetic ratio depends on mass. Ground state of hydrogen split depending on singlet/triplet state (triplet higher energy).
H Atom Pert, Which correction responsible for 21cm line.
Hyperfine Structure.
Eigenvalues of Spin-Orbit Operator.
When there are definite values of j, l, and s.
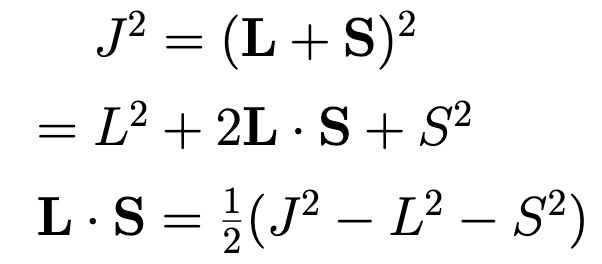
Shell Model Notation?
n - energy shells, l - orbitals
s , l = 0
p, l = 1
d, l = 2
f, l = 3
Shell Model, how many possible orbitals and states?
Possible ORBITALS (in each energy shell): 2n²
Possible STATES (in each orbital): 2(2l + 1)
Shell Model, how do shells fill?
Preferring smaller values of l, up to Argon (18), where rules break down.
What atoms are chemically inert?
Nobel Gases (full energy shells, max amount of electrons).
What atoms have an “extra” electron?
Alkali Metals, tendency to shed an electron and become and ION.
What atoms have one “too few” an electron?
Halogens, want to gain one, accounts for salts (ionic bonds).
What is Stark effect?
ELECTRIC FIELD, splitting of energy levels into closely spaced multiplets.
What is Zeeman effect?
MAGNETIC FIELD, splitting of energy levels into closely spaced multiplets.
Stark effect Hamiltonian?
For small |E| and r, can be treated as perturbation.
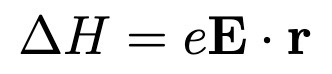
Stark effect change in ground state energy of hydrogenic atom?
None
Magnitude of splitting (Stark Effect)
Hydrogen d = a0
First order energy shifts start happening at n = 2.
m = +- 1: unperturbed
2s and 2p with m = 0: perturbed
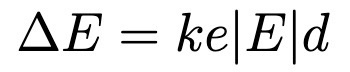
Zeeman effect Hamiltonian.
e/2m: classical gyromagnetic ratio
2 in front of S is because quantum gyro ratio is 2x classical.
Stern-Gerlach Experiment?
Zeeman effect, magnetic field splits a beam of atoms in two, measures mj. Splitting into two sharp components showed that spin was quantized.
Zeeman Effect (small |B| and large |B|)
Small |B|: Treat Zeeman Hamiltonian as a pert on top of fine structure.
Large |B|: Treat Fine Structure as a pert on top of Zeeman Hamiltonian.
What do Selection Rules describe.
Emission and absorption of electromagnetic radiation by atoms.
Going from Psinlm to Psin’l’m’
Uses Electric Dipole approximation (wavelength of light is much larger than atom so they feel a homogenous electric/magnetic field).
Selection Rules?
No transitions occur UNLESS delta m = +- 1 or 0
No transitions occur UNLESS delta l = +- 1
Rules don’t allow 2s to 1s transition, but get around them because of two-photon emission. (If you shine light on Hydrogen, first excited states will populate 2p (l = 1)).
What is a Blackbody?
Absorbs ALL radiation
Reflects NO radiation
Any radiation emitted comes from its temperature
What is ultraviolet catastophe?
Black body power spectrum goes as omega³. Power would grow without bound as frequency increased.
Plank introduced second term (Bose-Einstein factor) for identical photons.
Stefan Boltzmann Law
Power goes as T4
T - temp
P - Power
A - Area
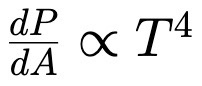
Wien’s Displacement Law
Location of Blackbody spectrum peak.
lambda max = (2.9 × 10-3 K ; m)T-1
Complex Conjugate
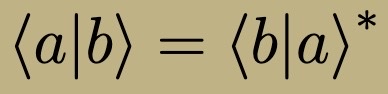
Hermitian Operator
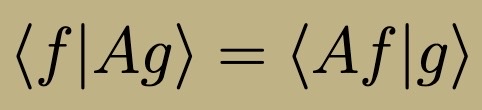
Expectation Value of an Observable
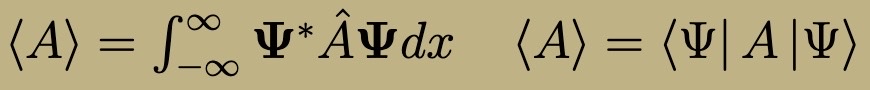
Probability of finding particle between x and x+dx
Complex Modulus
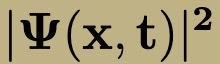
Position and Momentum Operators
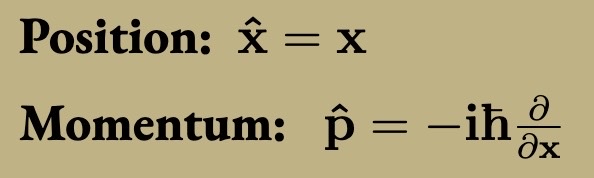
Schrodinger Equation
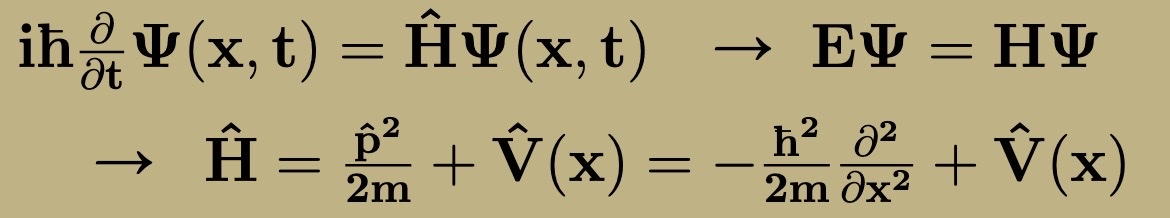
Steps for finding possible energies of system
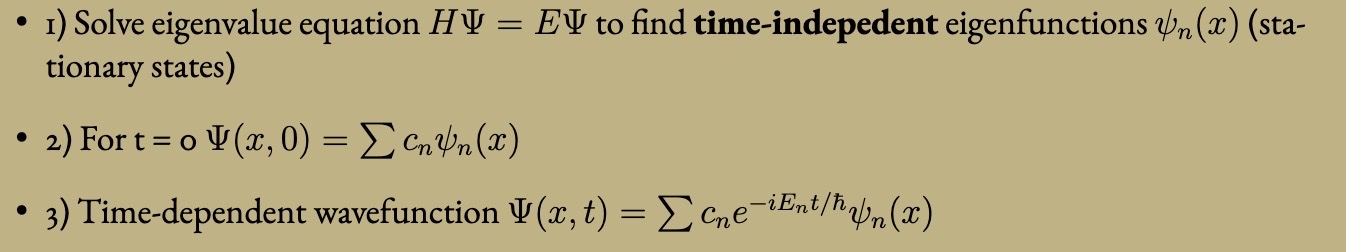
Retaining normalization, how can the wavefunction be altered.
Phase Shifted eiθ (cancels out with Psi2).
Operator that returns same function multiplied by constant c.
f(x) is eigenfunction of operator O.
c is eigenvalue
Hermitian Operator Properties
All eigenvalues are real
Eigenfunctions with different eigenvalues are orthogonal (integral f(x)* g(x) = 0)
Dimension of wavefunctions.
length-d/2 for d spatial dimensions
Expectation Value of observable A (decomp in an orthonormal basis)
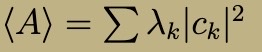
What is Hilbert Space?
Where kets live (a vector space in which inner-products may be taken)
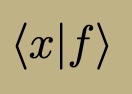
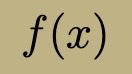
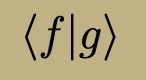
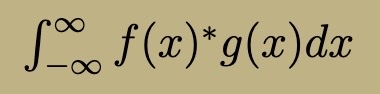
Time-Independent Energy Eigenfunction Properties
Psin for different n are orthogonal (different eigenvalues)
Psi is continuous
Derivative of Psi (with respect to x) is continuous EXCEPT at boundary where V(x) is infinite
Psi is purely real
Psin expectation value of momentum p is 0
How many nodes for each quantum energy state
Psi0 - 0 nodes
Psi1 - 1 node
Psi2 - 2 nodes
What condition of a potential V(x) must be met for definite parity
V(x) must be even
Definite Parity: Psi is either even or odd
Even psi(x) = psi(-x)
Odd psi(x) = -psi(-x)
Ground state even, first excited odd, second excited even and so on
What condition of V(x) must be true for the wavefunction to be normalizable.
E > Vmin (else kinetic energy would need to be negative which is impossible)
Do Operators commute in general?
No (applying A and then B is not typically the same as applying B and then A)
Canonical Commutation Relation
[x, p] = ih
Commutators of Products of Operator Rules
[AB, C] = A[B,C] + [A,C]B
[A, BC] = [A,B]C + B[A,C]
Uncertainty Principle (Commutation Relation)
<[A,B]> means uncertainty bound depends on what state the system is in
When inequality becomes a equality, this is the minimal uncertainty bound
![<p><[A,B]> means uncertainty bound depends on what state the system is in</p><p>When inequality becomes a equality, this is the minimal uncertainty bound</p>](https://knowt-user-attachments.s3.amazonaws.com/21d60674-8d4e-443a-a1be-c657b59a0a74.jpg)
Uncertainty Principle
σxσp ≥ h/2
∆x∆p ~ h
∆E∆t ~ h (short decay lifetime means widely varying total energy)
An observable is conserved when?
Its operator commutes with the Hamiltonian
We can diagnolize O and H
Hamiltonian for quantum Harmonic Oscillator
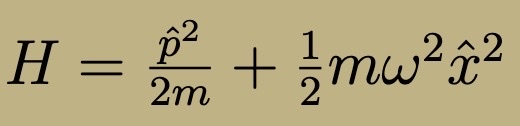
Hamiltonian Quantum Harmonic Oscillator (raising/lowering operators)
a dagger: raising operator
a: lowering operator
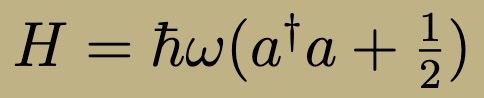
Ground state energy for harmonic oscillator
Also a minimum-uncertainty state
position-wavefunction is gaussian
width of gaussian is (h/m omega)^1/2
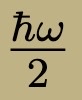
Energy spectrum of quantum harmonic oscillator-
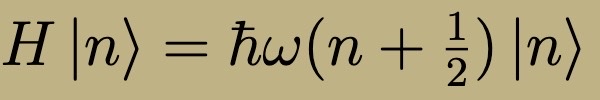
Harmonic Oscillator position-space wavefunction are related to what kind of polynomials?
Hermite Polynomials
Virial Theorem
Obeyed by all energy eigenstates of quantum harmonic oscillator
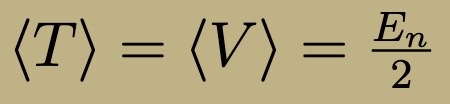
General Rules for 3D Quantum Harmonic Oscillator
Energy eigenfunctions: products
Energies: sums
Ground State: Non degenerate, First excited: three fold degeneracy
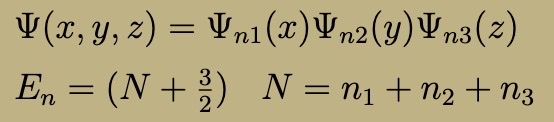
Bound Vs. Scattering States
Bound: E < 0 (LESS)
Scattering: E > 0 (GREATER)
If potential goes to infinity, every state is bound
Infinite Square Well Hamiltonian, Wavefunction, Energies
Wavefunction continuous at boundary
Wavefunction derivative NOT continuous at boundary
n starts at 1 NOT 0
Double mass- half the energy
Double well size - energy changes by 1/4
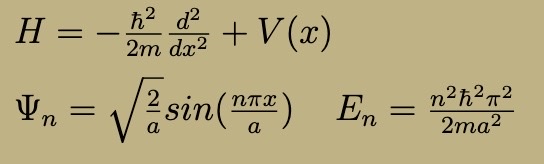
Free Particle Hamiltonian, Wavefunction, and Energies
Energy: p²/2m, de Brogile formula p = hk
Free particle can have any momentum at all (no boundary condition to restrict k)
Energy eigenfunctions are NOT normalizable
Create normalizable wavefunction by forming continuous wavefunctions with different k (wave packet)
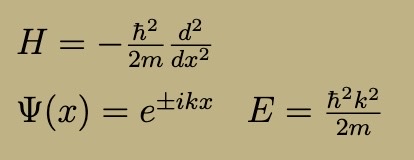
Delta Function Hamiltonian, Wavefunction, and Energy
A has dimensions of energy x length
Delta Function has only one bound state
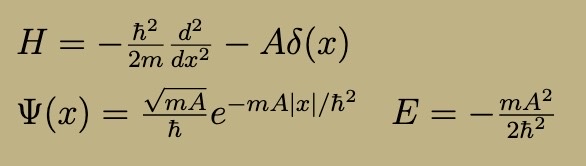
Finite Square Well
Outside well, where V = 0, solutions are the same as the free particle (normalizable one is e^kx)
Inside well, solutions are oscillating sins and cosines
Potential is even, ground state is even
As potential gets shallower and shallower, excited states disappear one by one until only one bound state remains
3D Hamiltonian
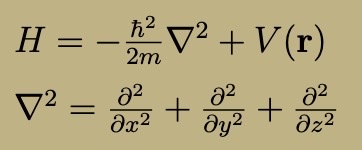
3D Commutation Relations
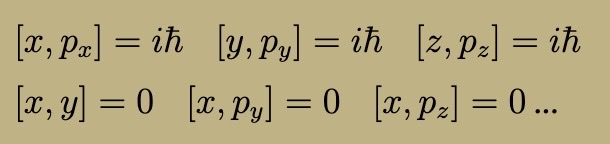
Free-Body Forces
Fair - air resistance
Fp - applied force
Fkf - kinetic friction
FN - normal force
Fs - spring force
FT - tension force
Fsf - static friction
Kinematic Equations
∆x = vi t + ½ a t²
vf² = vi² + 2a∆x
vf = vi + at
∆x = (vi + vf) / 2 t
2D Kinematic Equations
x(t) = v0x t + x0
y(t) = -½ gt² + v0y t + y0
vyi = v sinθ
What to use for straight up/down projectile
Conservation of energy
mgd = ½ mv²
Circular Motion
Centripetal Force
atan = 0
vtan = constant
arad = v²/r
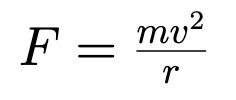
Conservative Vs Non-conservative Force
Conservative (T + U = Constant): gravity, spring, electric
Non-conservative: friction
Translational / Rotational Kinetic Energy
Translational: ½ mv²
Rotational: ½ Iω²
Gravitational / Spring Potential Energy
Gravitational: mgh
Spring: ½ kx²
Gravitational Force
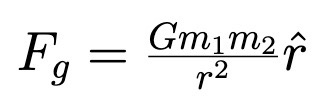
Angular Momentum (point particle and extended body)
Point Particle: L = r x p
Extended Body: L = Iω
Work Energy Theorem
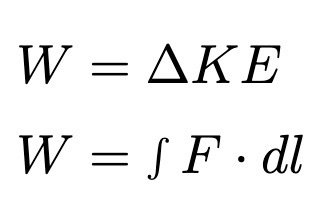
Velocity of block at bottom of curve
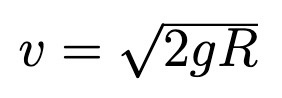
Rolling Without Slipping (velocity / energy)
solve everything in terms of v
friction does no work, diverts translational into rotational energy
I goes as m
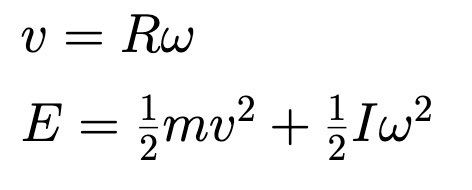
Torque
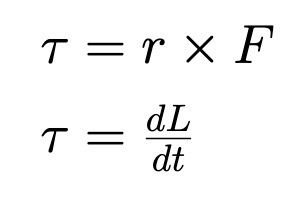
Radial Acceleration
Under Uniform Circular Motion (no tangential acc, constant tangential velocity)
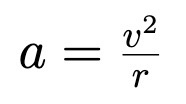
Reduced Mass
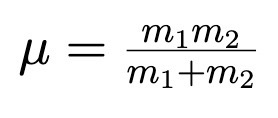
Change in Potential Energy between points a and b
When the force F is conservative.
Also means F = - nabla U
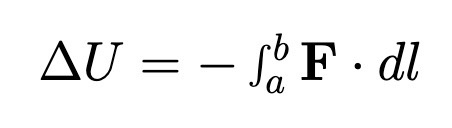
Centrifugal Force
Omega is constant angular velocity (not-inertial)
Not a real force!
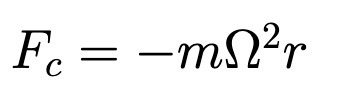
Coriolis Forc
Omega is constant angular velocity (not-inertial)
Not a real force!
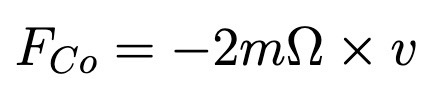
Moment of Inertia (Point Particle, Extended Object)
Weights mass by square of distance from axis of rotation
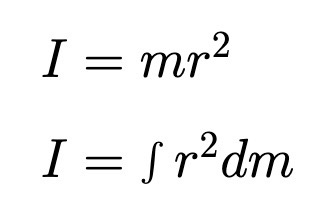
Parallel Axis Theorem
For moment of inertia of about any axis parallel to the CM axis.
Ex. moment of inertia penny rotating around an axis that passes through the edge of the penny
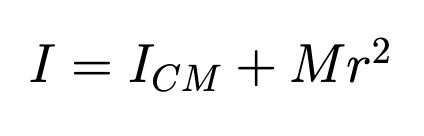
Center of Mass (Extended Object and System of Point Masses)
Weights the mass by DISPLACEMENT from origin
First one is really a triple integral (one for each coordinate)
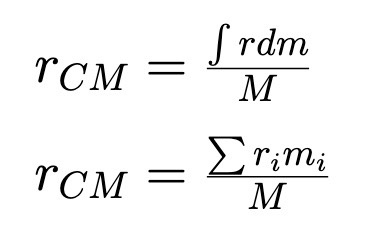
Lagrangian
Kinetic MINUS Potential
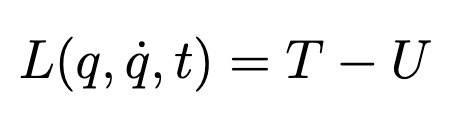
Hamiltonian (Classical)
Kinetic PLUS Potential
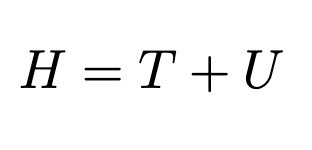
Kepler’s Laws
Planets move in elliptical orbits with one focus at the sun.
Planetary orbits sweep out equal areas in equal time.
T is period of planetary orbit, a is semi-major axis, k is constant same for all planets
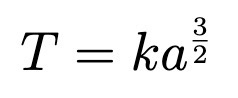
Hooke’s Law
x is displacement of spring from equilibrium
k is spring constant
Describes the harmonic oscillator
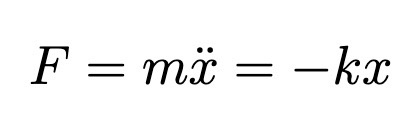
Harmonic Oscillator Solution Form (Classical)
A is amplitude, not determined by Hooke’s law but instead by initial conditions
Phi is the phase
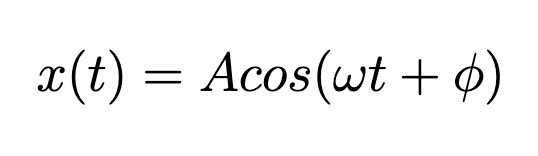
Angular Frequency of Harmonic Oscillator
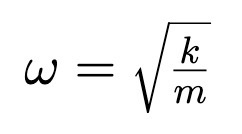
Spring Potential Energy
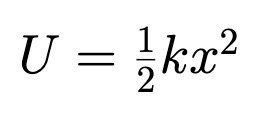
Secular Equation (Normal Modes)