Biology Unit 3 Energy Flow Concepts 1,2 & 3
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
31 Terms
Metabolism
All of the chemical reactions within each cell of an organism
Chemical reactions
The breaking and forming of bonds between different substances during chemical changes.
Catabolic
Reactions that break down larger molecules into simpler compounds
Anabolic
Reactions that build larger molecules from smaller ones
Activation energy
The amount of energy needed to make a chemical reaction start
Reactant (substrate)
Substance that is changed during a chemical reaction
Product
Substance that is made during a chemical reaction
Endothermic
A reaction that overall absorbs more energy (in the form of heat or light) than it releases
Exothermic
A reaction that overall releases more energy (in the form of heat or light) than it absorbs
Enzyme
(Usually a protein) that speeds up a biochemical reaction by lowering its activation energy
Catalyst
Substances that speed up reactions without being permanently altered
Active Site
Location on an enzyme where the substrate binds
Denaturation
When an enzyme's active site loses its specific shape, causing a loss in biological activity
Summarize how energy changes during a chemical reaction as bonds are broken and formed
Energy is released when bonds are formed.
Energy is absorbed when bonds are broken.
All reactions require energy to be absorbed and released.
No energy in the system gets lost, but can seem lost due to a change in form.
Explain the overall function of enzymes in biochemical reactions like photosynthesis and cellular respiration
Enzymes control all metabolic reactions. They speed up reactions (that can occur on their own, just slowly) by lowering the activation energy needed to get the reaction started.
Describe five factors that affect the rate of chemical reactions.
As temperature increases, the rate increases (to a degree) due to molecules moving faster and colliding more.
Enzymes work in specific pH ranges. They can lose function outside of that range and not be able to speed up the reaction.
As substrate concentration increases, the rate increases, due to more particle collisions.
Catalysts (like enzymes) speed up reactions by lowering the activation energy needed.
Competitive inhibitors lower the rate by competing with substrate for the enzyme’s active site.
ATP
An energy-carrying molecule that carries/stores energy for cell functions; the main energy currency for the cell
Explain the purpose of ATP
ATP carries energy in a usable form for the cell wherever it is needed.
Draw the structure and label the parts of a molecule of ATP.
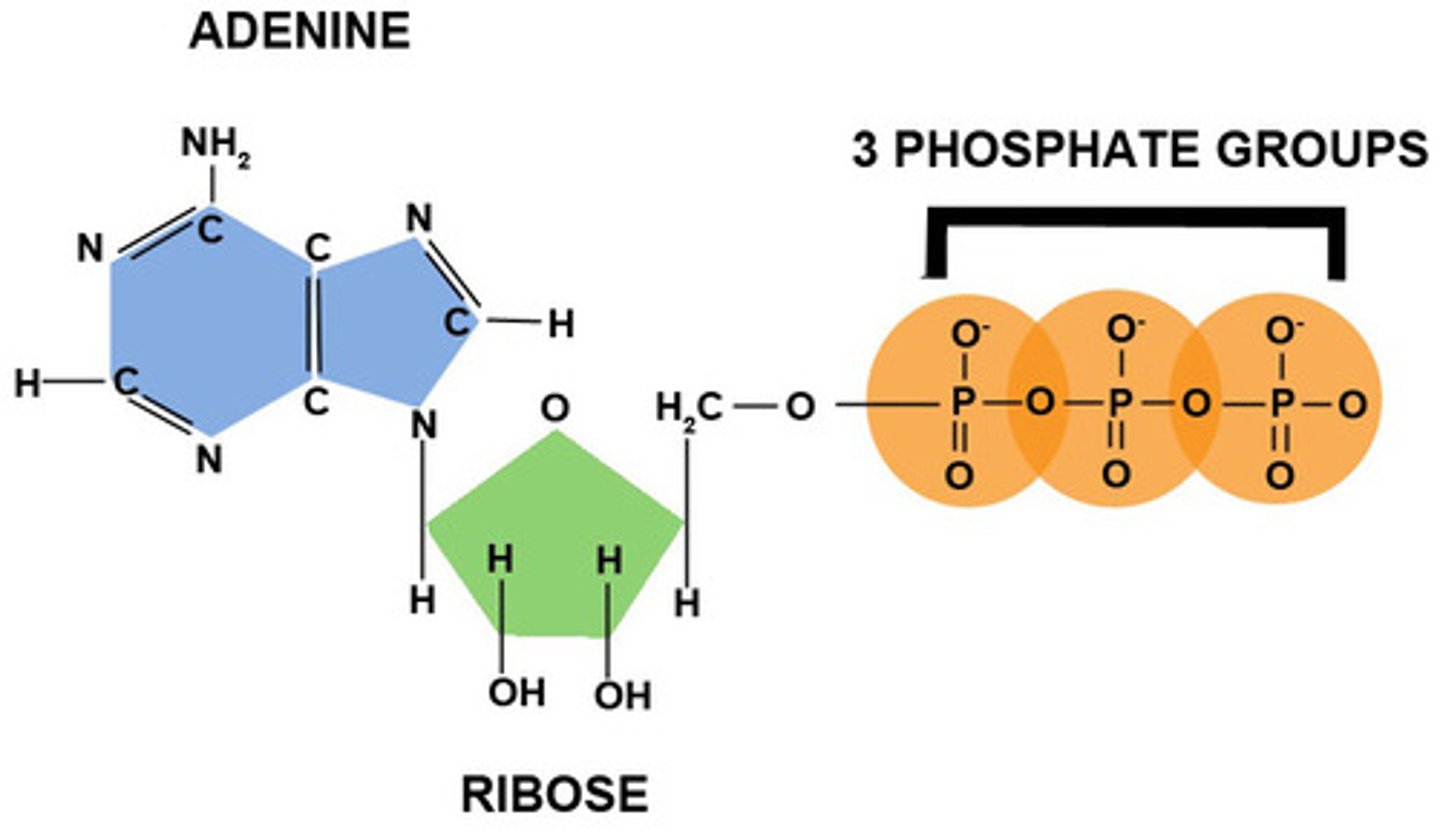
Describe the ATP-ADP cycle. Include what is and is not recycled.
ATP is really unstable, so it doesn't take a lot of energy to break the bond between the 2nd and 3rd phosphates.
When this happens and the P goes off to bond elsewhere, energy is released for the cell to use.
When consumed food gets broken down during cellular respiration, the energy gets stored in ATP by adding a 3rd phosphate back on to ADP.
ADP is recycled, nothing else necessarily is.
Explain what the energy is used for when a phosphate is removed, and where that energy initially comes from
Energy can be used for active transport, cell division, or other cellular processes.
That energy initially comes from glucose (a carb) and other macromolecules.
Summarize why the overall process of breaking down ATP is considered an exothermic process, while the overall process of forming ATP is considered an endothermic process
It doesn’t take a big input of energy to break the 3rd phosphate off (since ATP is so unstable). Overall, more energy is released than absorbed by this process, and thus it is considered exothermic.
It takes a lot of energy to attach the 3rd phosphate, so very little is released when ATP is formed. Overall, because more energy is absorbed than released, it is considered an endothermic process.
Producer
Autotroph; gets energy from nonliving sources like the sun
Consumer
Heterotroph; gets energy from living or once-living sources
Detritivore
Type of consumer that gets energy/nutrients from dead matter
Carnivore
Type of consumer that gets energy from eating meat
Omnivore
Type of consumer that gets energy from eating meat and vegetation
Herbivore
Type of consumer that gets energy from eating vegetation
Explain the difference between how autotrophs and heterotrophs acquire energy
Autotrophs are self-nourishers, and thus make their own food sources from nonliving sources, like the sun, or chemicals.
Heterotrophs are "other-nourishers", and thus they consume other organisms (or once living organisms) for food since they can't make their own.
Explain the significance of detritivores (decomposers) in a food chain/food web.
They play a critical role in returning nutrients from dead matter back to the soil when they poop. Plants can then absorb these nutrients.
Explain why the pyramid shape is used to represent energy, biomass, and numbers pyramids.
Due to the “rule of 10”, only 10% of the energy available gets passed on to each subsequent level in a trophic pyramid. 90% is either used by the organism or ”lost” as heat.
Because of this, less and less energy is passed on.
In order for an ecosystem to maintain stability, you also need the majority of organisms to be producers (thus the wider base) and the least amount of organisms (whether by biomass or numbers) to be top level consumers (thus the narrow top of the pyramid).