💊Compounding EXAM 1 Study Guide
1/149
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
150 Terms
Primary Packaging
💊What does the USP require?
💊Compound preparations must be package in a container that meet with the USP standards
💊 The container depends on (2)
💊Chemical properties of the preparation
💊Physical properties of the preparation
Container
💊Define
💊 Consists of 2 parts
💊The object that holds the compound preparation or is in direct contract with it
💊Immediate container
💊Closure
Immediate container
💊The container that comes in direct contact with the compound preparation
Is the closure part of the container ?
💊Yes
The 4 Type of Containers
💊Light resistance container
💊Well-closed container
💊Tight container
💊Hermetic container
Light Resistance Container
💊Define
💊Abbervation
💊Conatiner that protects the preparation from light effects
💊LR
Well-Closed Container
💊Define
💊Material
💊Abbervation
💊This container protects the preparation from extraneous solids and from loss of preparation during handling, shipment, and storage
💊Glass or Plastic
💊W
Tight Containers
💊Define
💊Fact
💊Material
💊Abbervation
💊This container that protects the preparation from contamination, loss of preparation, evaporation, efflorescent, evaporation and deliquescence due to handling and storage.
💊 Capable of tight closure
💊Glass
💊T
💊Glass
Hermetic Containers
💊Define
💊Fact
💊This container is impervious to air and other gases during handling & and storage
💊 Capable of tight closure
💊What will happen if you flip a well-closed container upside what will happen?
💊What will happen if you flip a tight container upside?
💊The product will spill
💊The product will NOT spill
💊What do Well-closed containers & Tight containers was in common ?
💊 Their both also light resistant
💊What type of glass are Parental drugs stored in ?
💊Glass
Freezer
💊What is the Degree in Celsius
💊Between -25 to -10
Cold
💊What is the Degree in Celsius
💊Between 2 to 8
Cool
💊What is the Degree in Celsius
💊Between 8 to 15
Room temperature
💊Temperature of the working area
CONTROLLED room temperature
💊What is the Degree in Celsius
💊Between 20 to 25
Warm
💊What is the Degree in Celsius
💊Between 30 to 40
Excessive heat
💊What is the Degree in Celsius
💊Above 40
Freezing point
💊32
Temp. of Refrigerator
💊Between 2 to 8
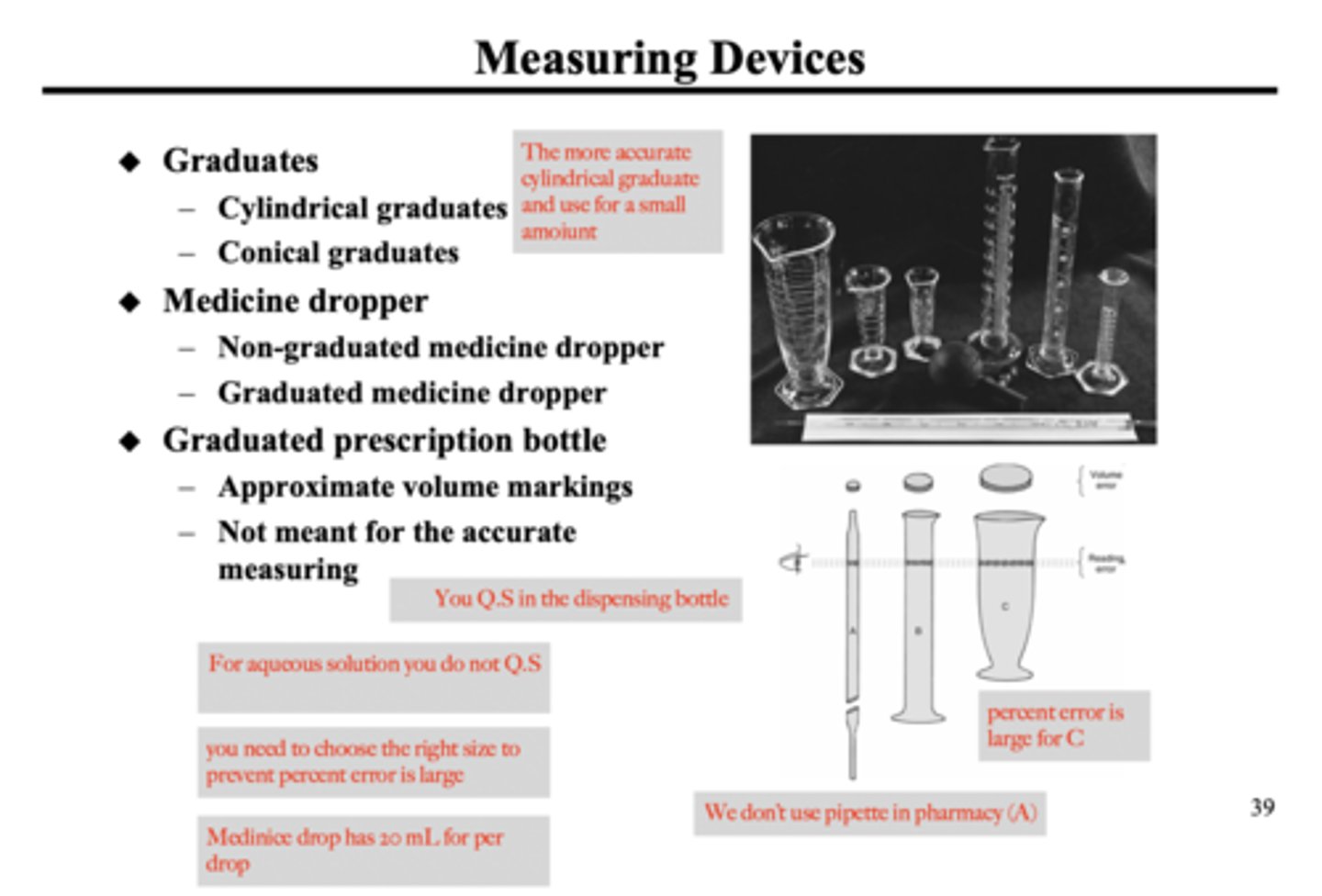
💊What type of water do you use to wash your dispensing bottle?
💊Purified water
💊What happens when your transferring a preparation from you measuring device to container ?
💊When you transferring a non-adhesive (simple syrup) to container, you lose some of your preparation when transferring. Now the medication asks for exactly 120 mL of this drug, but you loss some preparation when transferring from the measuring device to the container, what you have to do is Q.S the medication to 120mL
💊YOU NEVER Q.S on the measuring device, you only Q.S in the dispensing bottle
💊How many time should you wash you container
4 to 5 times
BUD (ON EXAM)
💊Stands for
💊What is it?
💊When is BUD determined?
💊Law
💊Fact
💊Beyond used date
------------------------------
💊Its the date in which the compound preparation should not be used
------------------------------
💊Determined the day the preparation was made
------------------------------
💊The label or package MUST HAVE A BUD (FEDERAL LAW)
------------------------------
💊BUD is not the same as expiration date
Nonaqueous Formulation (NON-STERILE)ON EXAM)
💊How is the BUD determined?
💊BUD should not be later than the earliest expirations of API (active ingredient) or 6 months (which every is earlier)
★ The professor wants you to mostly focus on BUD for non-sterile
Water-containing Oral Formulation
💊How is the BUD determined?
💊Example
💊BUD is not later than 14 days since the medication was stored in cold temp.
💊Suspension
💊What is the difference between BUD & and Expiration Date?
💊 Expiration date is when the drug potency reaches 10%
💊BUD source is the API (active ingredient) expiration
💊Expiration Date
💊 Expiration date is when the drug potency reaches 10%
Water-Containing Topical/Dermal & Mucosal Liquid & Semisolid Formulation
💊How is the BUD determined?
💊The BUD is not later than 30 days
💊If a non-sterile preparation has an expiration date is 1 year, what should be the BUD ?
💊BUD should be 6 months.
★ BUD for non-sterile preparation must be 6 months or the earliest expiration of API. In this question it says the expiration Is 1 year which is longer than 6 months, so were going to put the BUD as 6 months
💊If a non-sterile preparation has an expiration date is 6 months, what should be the BUD ?
💊BUD should be 6 months
★ BUD for non-sterile preparation must be 6 months or the earliest expiration of API.
💊If a non-sterile preparation has an expiration date is 1 months, what should be the BUD ?
💊BUD should be 1 month
★ BUD for non-sterile preparation must be 6 months or the earliest expiration of API. Here the API is 1 month, so were going to put the earliest expiration date of the API which is 1 month instead 6 months
💊If a non-sterile preparation has an expiration date is 7 months, what should be the BUD ?
💊BUD should be 6 months.
7 Major Caterogies of Compounding
💊Category 1 & 2 → Describes non-sterile compounding
💊Category 3,4,5 → Describe sterile compounding & risks
💊 Category 6 & 7 → Radiopharmaceuticals & veterinary compuounding
The reason for Classifying categories of compounding
💊To provide the compounding personnel an understanding when different forms of preparations are compounded
💊The USP states that: "it is to be understood that there are levels of training associated with each category"
Category 1
💊Description
💊Examples
💊Non-sterile & Simple
💊Mixing 2 or more commercially products togethers
★ The professor what's you to only focus on the Non-sterile categories
Category 2
💊Description
💊Examples
💊Non-sterile & Complex
💊Compounding with a drug Bulk Or calculation are required
★ The professor what's you to only focus on the Non-sterile categories
Category 3
💊Description
💊Example
💊Sterile & Risk level 1
💊Low risk
Category 4
💊Description
💊Example
💊Sterile & Risk level 2
💊Medium risk
Category 5
💊Description
💊Example
💊Sterile & Risk level 3
💊High risk
Reference Resources (4)
★Just read through, it will NOT be tested on the exam (honestly idk why I put it here)
💊USP
★ For the entire U.S.A
💊FDA Regulations of Hospital compounding
★ Related to gov't & gives guidelines used by USP
💊Sate Regulations of Compounding
★Compounding is different for every state.
💊National Association of Boards of Pharmacy
💊 Can a pharmacy compound a product prior to receiving a prescription for it?
Yes, under certain restriction
💊 Pharmacist must compound the medication in response to a prescription (CANNOT BE DONE BEFORE HAND)
💊 Pharmacist must only compound a very small quantity of drug, ONLY the based on the history of the patient coming back multiple times to receive that particular medication
💊 Can a pharmacy compound drugs that were withdrawn or removed from the market for safety reasons?
No
💊 The pharmacy cannot compound a medication that goes against the enforcement of the FDA. SAME applies to dietary supplements removed from the market
💊 Is it permissible for a practitioner to post a date on a prescription for a controlled substance in lieu of issuing such a prescription on the date it is due?
(Simpler terms: The questions is says can a patient go to a pharmacy with a prescription that is might for December 9th, but their going to the pharmacy and asking for it on September 16 ;)
No
💊 Can a pharmacy compound drugs from bulk active ingredients that have never been approved for marketing in the United States?
No,
💊 The drugs need to be FDA approved
💊 This only applies to drugs under IND stage
💊 Can a pharmacist compound with any drug from any source, using any available source for its ingredient(s)?
No
💊 The ingredient must be USP (Ex you can't you use any lactose you need to use lactose USP approved)
💊Can a pharmacy receive, store, or use drug substances or drug components not guaranteed or otherwise determined to meet official compendial requirements?
No
💊Ingredients must be align with USP/NF monograph
💊If the USP/NF monograph does not exist for the ingredient then the ingredient must be approved by Secretary of Health and Human services
💊Can a pharmacy use commercial-scale manufacturing equipment for compounding drug products?
No
💊We need a patient relationship and we don't use a bulk amount
💊Can a pharmacy advertise its compounding services?
Yes
💊Pharmacy can advertise its compounding services, BUT NOT THE DRUG PRODUCT ITSELF
💊 Can a pharmacist compound products similar to those that are commercially available in the marketplace or that are essentially copies of FDA-approved drug products?
No
💊Compounded product must be substantial different compared to a commercial product that get manufacturer
💊 Can a pharmacy compound drugs for third parties who resell to individual patients?
No
⊛VS
⊛FS
⊛Sol
⊛SpS
⊛SIS
⊛VSS
⊛PartSol
⊛PrIn
⊛Is
⊛Misc
⊛Part Misc
⊛Immisc
⊛Disp
⊛Swells
⊛Very soluble
⊛Freely soluble
⊛Soluble
⊛Sapringly soluble
⊛Slighty soluble
⊛Very slightly soluble
⊛Partially soluble
⊛Partically insoluble
⊛Insolube
⊛Miscible
⊛Partically Miscible
⊛Immiscible
⊛Dispersible
⊛Swells
These are some of the Abbreviations on the txtbook, there were way too many, so I put the common ones
⊛A
⊛aa
⊛a.c
⊛Ad
⊛Baln
⊛baln.vap
⊛b.i.q
⊛ C
⊛ CC
⊛ Coch.ampt
⊛ Coch.parv
⊛D
⊛DTD (d.t.d)
⊛ et
⊛ Gt, Gtt , guttat
⊛ H.d
⊛ HS (H.S)
⊛ In d.
⊛ in vitro
⊛ Noct.
⊛ Non rep.
⊛ O.D
Do these aberration on another day
⊛ Before
⊛ Each
⊛ Before meals
⊛ Up to
⊛ Bath
⊛ Steam bath
⊛ Twice a day
⊛ With
⊛ With meals
⊛ Tablespoonful
⊛ Teaspoonful
⊛ day
⊛ give of such doses
⊛ and
⊛ drop, drops, drop by drop
⊛ at bedtime
⊛ Before sleep
⊛ In a day
⊛ In glass
⊛ At night
⊛ Do not repeat
⊛ Right eye
Recommended Practices
All written prescription or medication orders must be legible
Prescribers should
All written prescription or medication orders must be legible
Prescribers should avoid the use of abbreviations
All prescriptions and medication orders should be written using the metric system
Prescribers should provide the age and, when appropriate, the weight of the patient on the prescription order
The prescriptions or medication order should include the name of the drug, metric weight or concentration, and dosage form
A leading zero should always precede a decimal point in quantities less than one
A trailing zero should never be used after a decimal point
Prescription and medication orders should include, when possible, a notation of purpose of the medication
Prescribers should not use unclear or imprecise instructions such as “Take as directed” or “Take as needed”
Writing numbers
💊When writing a decimal, remember to add a Zero in front of the decimal point (ex: 0.25 mg (good) → .25 mg (bad)
💊When writing a whole number, never added a decimal point and a zero behind the zero (ex: 25 mg (good) → 25.O mg (bad)
💊Put spaces between words, unit abbreviations, or set numbers (ex: acetaminophen 600 mg (good) → Acetaminophen 600mg (bad because their is no space between 600 and mg)
💊What is the weight sensitive Sensitivity requirement of Prescription Balance ? (ON EXAM)
💊 6 mg (This mean that the balance weight as little as 6 mg)
💊What is the weight-sensitive Sensitivity requirement of Top-Loading Balance ? (ON EXAM)
💊 1 mg (This means that the balance weight is as little as 6 mg)
💊Avoid errors of 5 % or more (do not weigh less than 20 mg, if you weight a compound less than 20 mg then you will have higher than 5% error)
Percentage of Error
💊Define:
💊Formula
💊 Maximum potential error expressed as a percentage
★ Quantity weighted = It the amount of drug that was actually measured ( the correct weight)
★ Quantity Desired = The quantity the prescription or the pharmacist ask for
★ Error is the Quantity weighed-Quantity desired

Let me know if its important to know about this and balances

Least Weighable Quantity
💊Define:
💊Formula
💊The minimum quantity that can be weighed with the desired degree of accuracy
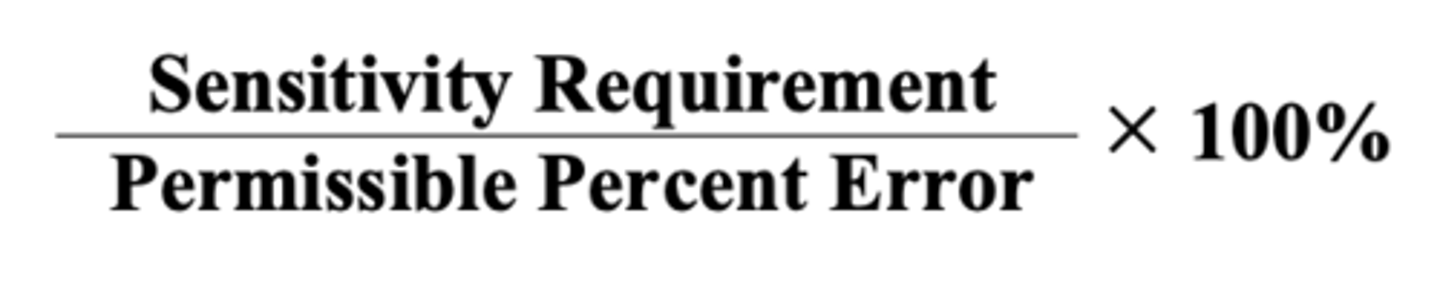





Compounding Powders
💊Consists of 2 substances
💊2 Types of Powder
💊API (Medicinal substance)
💊Excipients
------------------------
💊Internal use
💊External use
API
💊 The 2 forms
💊 Depends on the
💊 Crystalline
💊 Powdered Forms (better due to similar particular size)
------------------------
💊 Source and nature of the drugs
💊 Function "Sieve opening"
💊 Controls the particular size
💊 D50
💊 50% or more of the powdered material passes
💊 Understand that HIGHER the mesh number = The smaller particular size
💊 Understand the larger particulars = the smaller the surface area
💊 Understand the smaller particulars = the larger the surface area
💊 The surface area will increase and will able to control dissolution rate of water in drug this allows increase of bioavailability & more drug will be absorbed through the peripheral circulation, the solubility of the thing does not change because we didnt change the drug

💊Pharmaceutical elegance →
💊 Forming a homogenous mixture
Particle Size Reduction
💊The 4 Methods:
💊Purpose
💊Spatulation
💊Trituration
💊Levigation
💊Pulverization by intervention
💊To reduce the particle size of a small quantity of drug
Spatulation
💊Function (2)
💊The 5 Types of Spatulas
💊Particle size reduction only in soft particles
💊Only breaks soft agglomerate that are already in a fine state of subdivision
-----------------------------
💊Large Spatula
💊Small Spatula
💊Meta Spatula
💊Hard-rubber Spatula
💊Teflon-coated stainless steel Spatula
Advantages and Disadvantages of Extemporaneously Prepared Powders

💊Large Spatula : What is it used for ?
💊Levigating larger quantities of drug
Small Spatula
💊Best suited for →
💊 Purposes (3)
💊Dry Chemicals
💊Scraping materials from other spatulas
💊Scraping materials from mortars and pestles
💊 Levigating small quantities of drugs & chemicals on an ointment slab
Metal Spatulas
💊IMPORTANT FACT
💊 Metal Spatulas can not be used when handling drugs/substances that react metal because it can damage spatula or degrade/decompose the drug/substance (that comes in contact with the metal spatula)
💊List the metals substances that will react with Metal Spatulas
💊Iodine
💊Phenol
💊Acidic drugs ( salicylic acid & acetyl salicylic acid)
💊What should be used, instead of metal Spatula ?
💊Hard-rubber Spatula
💊Teflon-coated stainless steel Spatula
💊Example: Prescription comes and you have compound Iodine? What equipment would you use?
💊Hard-rubber Spatula
OR Teflon-coated stainless steel Spatula because Metal Spatulas that react metal and cause damage spatula or degrade/decompose the drug/substance (that comes in contact with the metal spatula)
Trituration
💊Explain the process:
💊Purpose (2)
💊Uses a mortar and pestle to grind the solid into a finer particles
💊Reduces particle size
💊Blends powder
Levigation
💊Explain the process
💊Important Fact (2)
💊Primarly used to
💊Alternative to
💊Blending powders with a small quantity of Levigating agent (minimal amount)
------------------------------
💊NOT an efficient method for particular size reduction
💊It does not reduce the particular size of hard or soft particulars (some may occur)
------------------------------
💊Incorporate a solid powdered into a ointment base
------------------------------
💊Trituration
💊Levigating agent (What is it ?)
💊Viscous non-solvent liquid
Pulverization by intervention
💊Explain Process
💊Purpose
💊List the substances that undergo Pulverization
💊Alternative to
#1 Mix the drug/substance with Volatile solvent (drops)
#2 Triturate the mixture until volatile solvent evaporates. (fine powder is left behind, and is air dried to ensure complete evaporation of the volatile solvent)
---------------------
💊Reduce particle sizes of substances that do NOT crush easily
---------------------
💊Hard crystalline substances
💊Gummy type substance
---------------------
💊Levigation
💊Hard crystalline substances (EX)
Iodine
💊Gummy type substance (EX 3)
💊Camphor
💊Menthol
💊Resorcinol
FACT about Iodine
💊Poor water soluble
💊Reacts with metal spatula
The 4 Types of Mortar and Pestle
💊Wedgwood
💊Glass
💊Porcelain
Wedgwood Mortar and Pestle
💊Purpose
💊Characteritisc
💊Comminution of hard crystalline solids
💊Porous
→ Wedgwood to stain easily
→ Small particle powder gets trapped inside the mortar
💊Porous causes ....?
→ Wedgwood to stain easily
→ Small particle powder get trapped inside the mortar
💊In what cases, should Wedgwood not be used
💊Potent drugs
💊Very small quantity of drugs
💊Drugs that stain
💊After continued use of a Wedgwood want tends to happen?
💊Wedgwood becomes smooth → losing its ability to communicate hard crystalline substance → we fix this by triturating a rough material (sand) to re-rough the mortar.
Glass Mortar and Pestle
💊Purpose
💊Characteritisc
💊Fact (2)
💊Disadvantages
💊Primarily used for preparing solution, suspensions and ointment that require the break down of soft aggregates
--------------------------
💊Smooth & Non-porous interior surface
--------------------------
💊Not suitable for grinding powder and crushing hard crystalline solids
💊Easy to Clean
--------------------------
💊Fragile and can break easily if pounded hard with pestile
💊Smooth and non-porous interior surface allows the use of (20
💊Drugs that stain
💊highly potent drugs
Porcelain Mortar and Pestle
💊Purpose
💊Characteritisc
💊Afavatnesg
💊Blending of powders of uniform size particles and fro comminution of soft aggregates or crystals
💊Exterior Glazed
💊Less porous
💊Good when you need posse and when large substances are used