Chemistry Chapter 5 - The Periodic Table
1/60
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
61 Terms
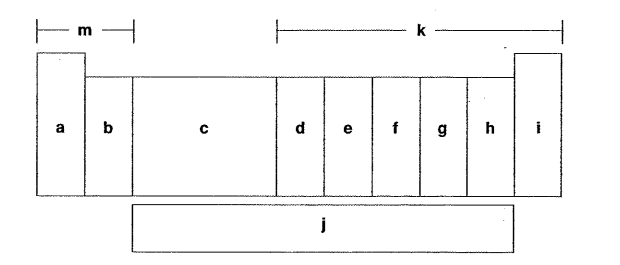
Which letter is the carbon family?
E
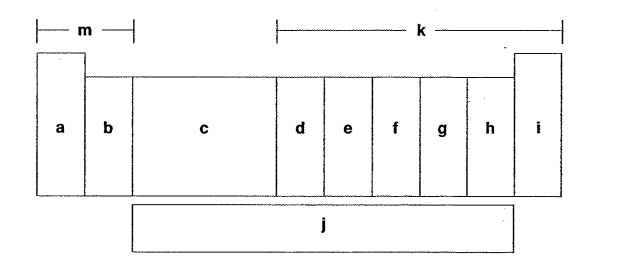
Which letter is the alkaline earth metals?
B
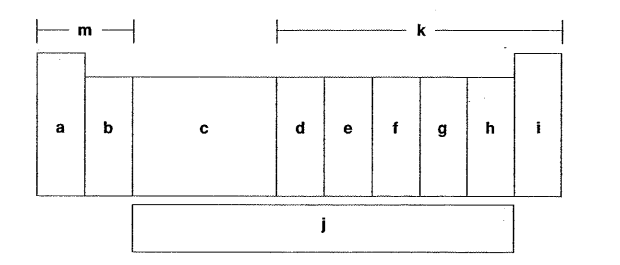
Which letter is the inner transition metals?
J
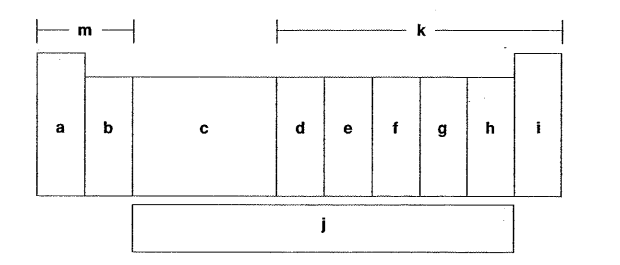
Which letter is the halogens?
H
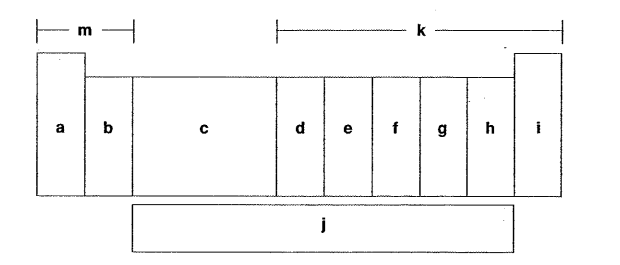
Which letter is the d-block elements?
C
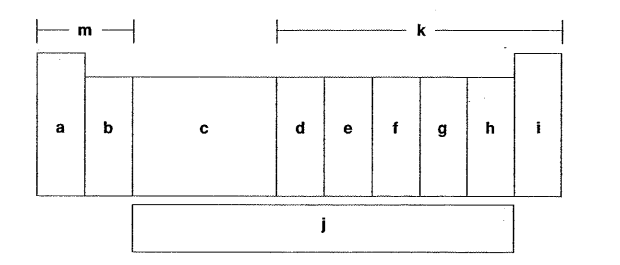
Which letter is the oxygen group?
G
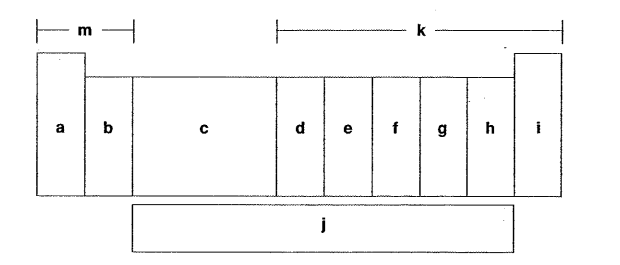
Which letter is the alkaline metals?
A
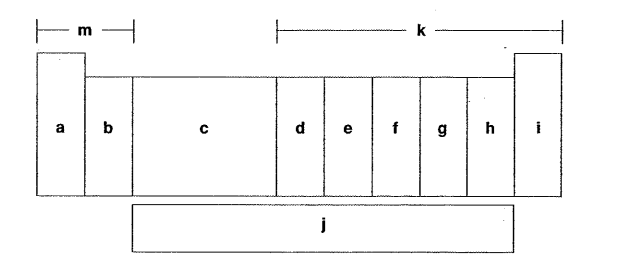
Which letter is the f-block elements?
J
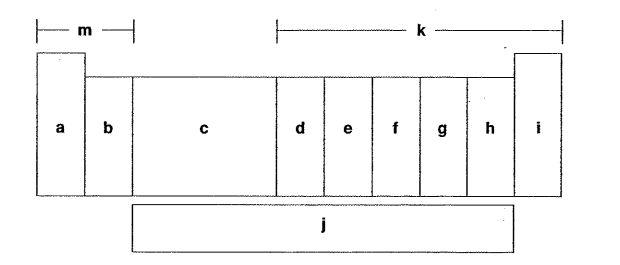
Which letter is the noble gases?
I
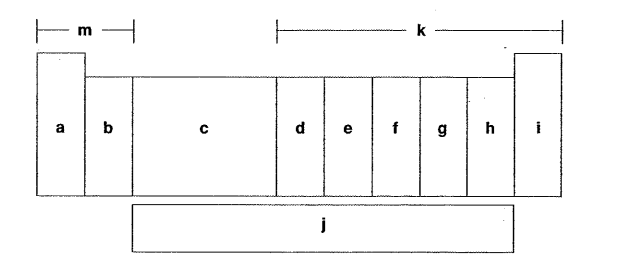
Which letter is the p-block elements?
K
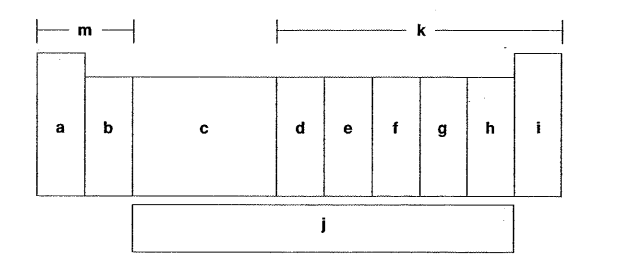
Which letter is the nitrogen family?
F
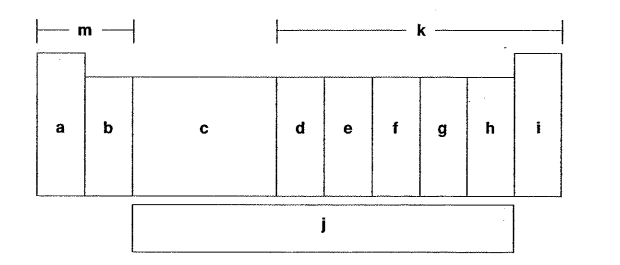
Which letter is the s-block elements?
M
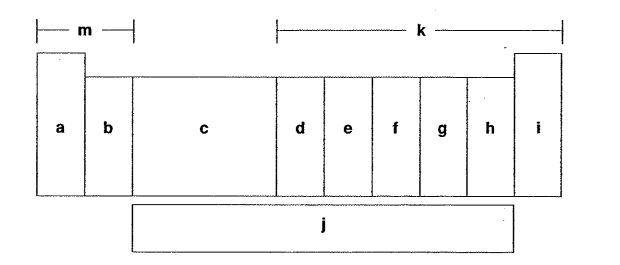
Which letter is the transition metals?
C
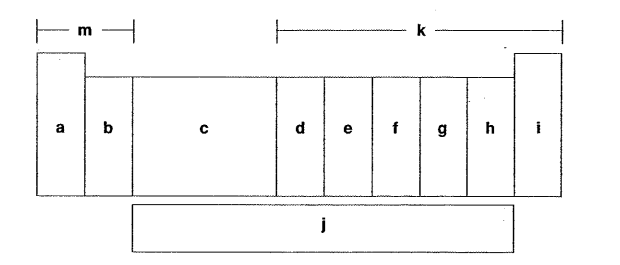
Which letter is the group of one sentimental and four metals?
D
What is an organized way to display the elements
The Periodic Table
How many elements were known in Early Chemistry
Early on in Chemistry only about 30 elements were known. These were easy to remember.
What scientist found that elements could be grouped
Dobereiner found that elements could be grouped.
What did Newland observe
Newland- observed that if elements were arranged in increasing atomic mass, the properties of every eighth element were the similar as the first.
What did Mendeleev do with the periodic table
Mendeleev- assembled elements on cards by increasing atomic mass.
What is Mendeleev known as
The father of the periodic table
Elements in the same _______ had ______ properties.
column; similar
How many elements are naturally occurring
92
What was wrong with Mendeleev's P.T.?
Mendeleev organized his by atomic mass instead of the correct way (atomic number)
Mendeleev broke the pattern sometimes in order....
Mendeleev broke the pattern sometimes in order to keep elements with similar properties in the same column.
Did Mendeleev give names to some of the empty slots in the Periodic Table
Yes, Mendeleev also gave names to some of the "holes" in the P. T..
What is the Periodic Law?
When elements are arranged in order of increasing atomic number, their physical and chemical properties show a periodic pattern.
Groups/families are found where on the periodic table
Vertical columns
Groups are labeled 1A-8A and 1B-8B
Where are periods found on the periodic table
Periods- Horizontal rows
Name for Group 1 or 1A
- Alkali Metals
Name for Group 2 or 2A
- Alkaline Earth Metals
Name for Group 17 or 7A
- Halogens
Name for Group 18 or 8A
Noble gas
Do metals lose or gain electrons
loses electrons (become + cations)
What is a physical characteristic of Metals (3)
1- have luster/shine,
2- malleable
3- ductile
Are metals conductive and if so what do the conduct?
conduct electricity and heat
At room temperature medals are usually ____
are usually solid at room temperature
Where are the metals found on the periodic table
found on the left side of the PT
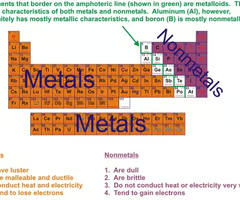
Do Nonmetals gain or lose electrons
gain
are nonmetals shiny or dull?
Do not have luster/dull
Are nonmetals good conductors of heat and electricity?
Poor conductors of heat and electricity
Are nonmetals malleable or ductile
Not malleable or ductile
What do nonmetals look like at room temperature
Either gases (mostly), liquids, or solids at room temp.
Where are nonmetals located on the periodic table?
Found on the upper right hand side of the PT
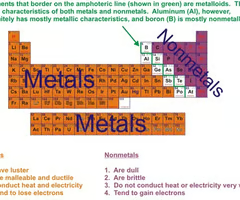
What is another name for semimetals
Also known as a metalloid
What category does semi metals fall under
Don't fit well in the metal or nonmetal category
What properties does semi metals have
Share properties of both metals and nonmetals.
What are Valence electrons
Electrons that are on the outermost shell.
What is responsible for an atoms chemical behavior
valance electrons
What group does valance electrons concern themselves with
Only concerned about the "A" group elements. (This means only 1A, 2A, 7A, 8A) Most likely because they are the easiet to have a full shell
What Tells where the electrons are at in the subshell.
s,p,d,f-blocks
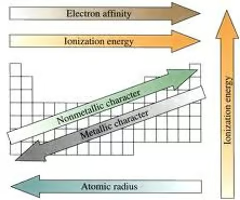
What is atomic radius
Atomic radius- distance from the center of an atom's nucleus to the outermost electron.
Atoms get larger going down a group. Why?
When you go down a column it adds rings, this adds to the mass of the atom
Atoms get smaller going from left to right. Why?
as you move across, the number of protons in the nucleus increases, which creates a stronger positive charge that pulls the electrons closer to the nucleus, effectively shrinking the atom's size
Ionization Energy
Defined as the energy needed to remove an electron from it's outershell.
Do all atoms want to give electrons
Some atoms will readily give up electrons and some will not.
If you want to remove a second electron, it requires ___ energy.
more
What is Electron Affinity/ Electronegativity
A measure of an atom's attraction for an extra electron.
Which element is the most electronegative?
Fluorine
Which element is the Least electronegative?
Francium
What is the goal for atoms
Goal for atom's is to have a full valence shell.
What are the trends in the periodic table
electronegativity, ionization energy, electron affinity, atomic radius, melting point, and metallic character