General chemistry 2
1/125
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
126 Terms

friction, drift and stokes law
particles in fluids lose energy due to friction, under a constant force, f a particle reaches a constant drift velocity Vd when friction balances the force
where e is the friction drag coefficient, constant for a given particle in a given medium and can be measured experimentally

for spherical particles stokes law applies
where n is the fluid visocity (10^-3 n/m²s for water) and r is the particle radius, larger particles have greater drag

flux and drift visocity
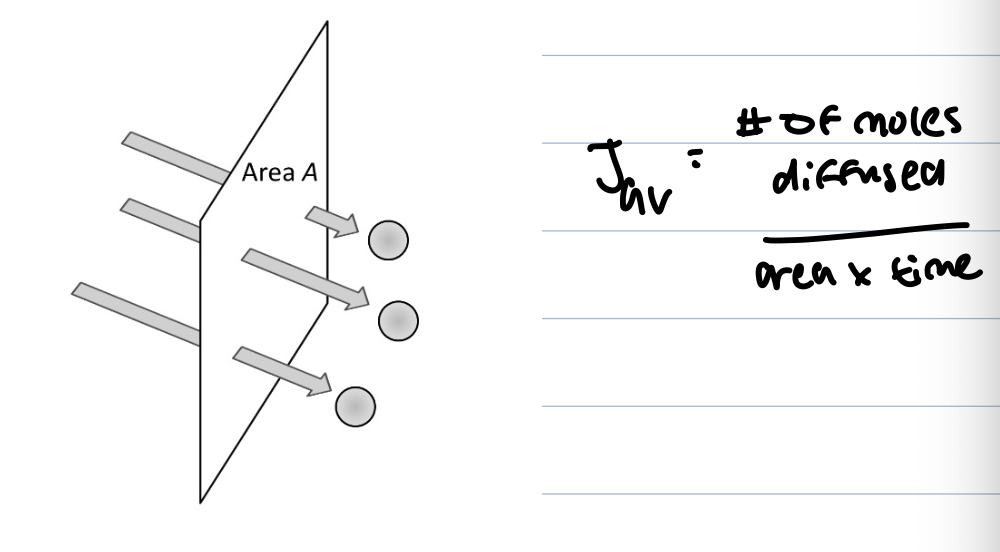
flux measures transported material per unit area per unit time, it is defined by counting particles crossing area A, in 1D flux J is above
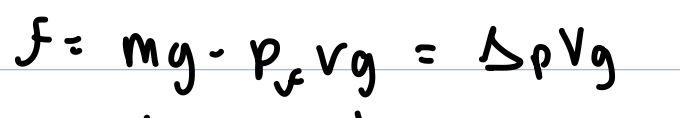
sedimentation
particles heavier than the surrounding liquid settle under gravity
applications occur in geology, water treatment
the netforce on a particle is gravity minus the fluid’s bouyant force
m= mass particle
g= 9.81
pf=density surrounding fluid
v= volume particle
change in p= difference in density between particle and fluid

sedimentation velocity
for spheres V= 4/3 pi r³
heavy particles move down, lighter particles rise, small particles sediment slowly so ultracentrifuges use centrifugal acceleration up to 10^5
change in p >0 then it moves downwards
change in p < 0 then it moves upwards
spontaneous spreading
diffusion
even without external forces, random thermal motion causes particles to spread from high to low concentration until uniform
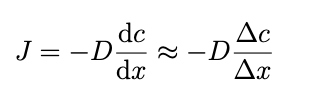
Ficks first law
negative sign ensures flux goes from high to low concentration (if concentration decreases in +x direction, gradient is negative giving positive flux)
D is diffusion coefficient around 10^-9 m²/s for small molecules in water, larger particles diffuse more slowly
J- diffusive flux (moles per unit area per unit time)
ac/ax- concentration gradient → conc. change/length
ion transport through a channel
membrane proteins forming narrow pores, a channel is approximated as a cyndrical tube
diameter d= 0.5nm
length L = 5nm (membrane thickness)
cells maintain concentration differences via active tranport
inside concentration 100mm greater than outside so potassium diffuses outwards

einstein relation
in most situations both directed motion (drift) and random motion (diffusion) occur simultaneously
drift arises from an external field, diffusion arises from conc gradients
Gas molecules in earth’s gravitational field
if molecules start uniformaly distributed, gravity pulls them downward (downward drift flux)
they accumulate near earth’s surface, creating a concentration gradient that produces an upward diffusive flux
total flux is sum of drift and diffusion
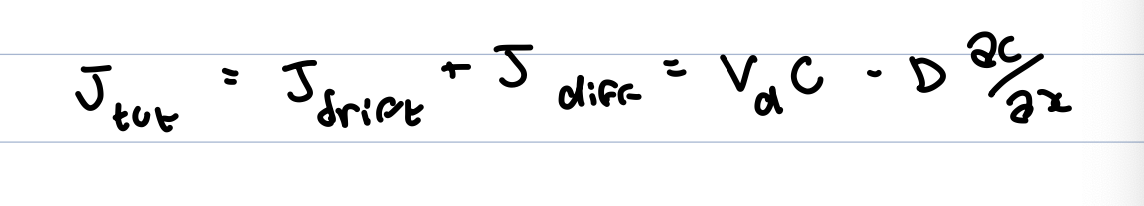
for gravity vd=mg/e
at equilibrium
nothing changes over time → Jtot=0

Einstein relation

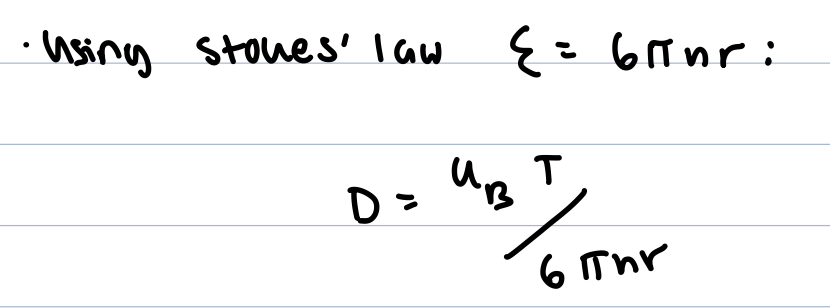
stokes einstein relation
this gives us diffusion coefficients for spherical ions, proteins, dirt particles, bacteria

brownian motion
caused by collisions with thermally agitated water molecules
random molecular motion also causes diffusion
diffusion can be viewed as the longterm outcome of a random walk
has uncorrelated steps, taken in random directions, but the particle is unlikely to be found far from where it started due to frequent reversals

t=x/v
t is time taken, v is velocity and x is distance to travel
this connects average distance travelled during diffusion eith the diffusion coefficient
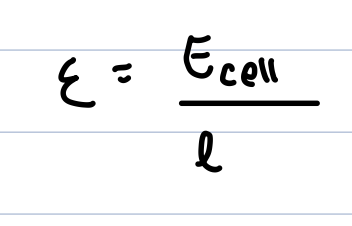
electric field setup
two parallel electrodes are placed distance l apart in a sample solution, applying a voltage Ecell creates an electric field
positive ions drift towards the negative electrode and negative towards the positive one
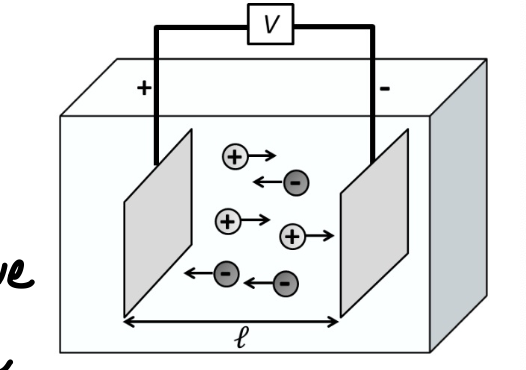
force on an ion
an ion with charge z e (valency z, elementary charge e=1.6 × 10^-19) experiences force-
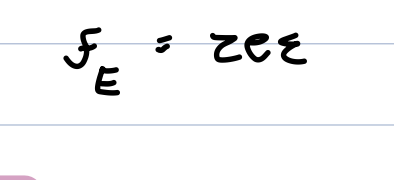
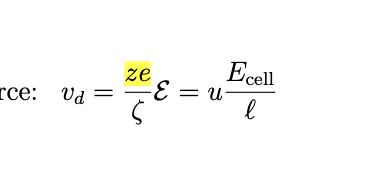
drift velocity
positive ions move one way z>0 and negative ions move the other

mobility definition
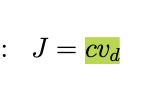
ionic flux under an electric field
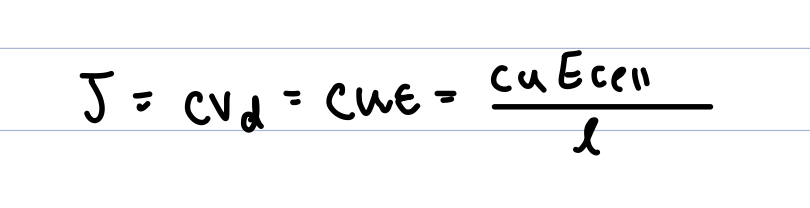

mobility in terms of stokes law
mobility depends on ionic charge, ionic radius and solvent visocity
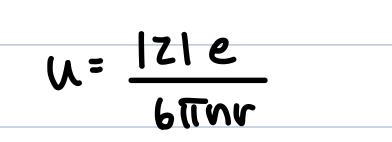
why Li+ has lower mobility despite smaller radius
stokes radius is the hydrodynamic radius which includes hydration
small ions have strong electric fields → attracts more water → thicker hydration shell → effectively larger radius → lower mobility

why H+ and OH- have very high mobility
protons move via the Grotthuss mechanism; bond rearrangments transfer charge without moving mass
OH- moves similairly
diffusion coefficient relation
since mobility relates to friction and friction to diffusion diffusion is linked to mobility
thus one mobility is known, D is easily computed

electrophoresis
macro ions migrate in an electric field
electrophoresis uses this to seperate biomolecules
migration depends on charge and size, protein charge depends on pH (acidic, alkaline groups protonated or deprotonated)
each protein has an isoelectric point where net charge is zero
when crossing this pH drift slows → stops → reverses
this allows seperation of protein mixtures
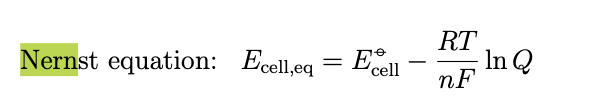
the nernst equation

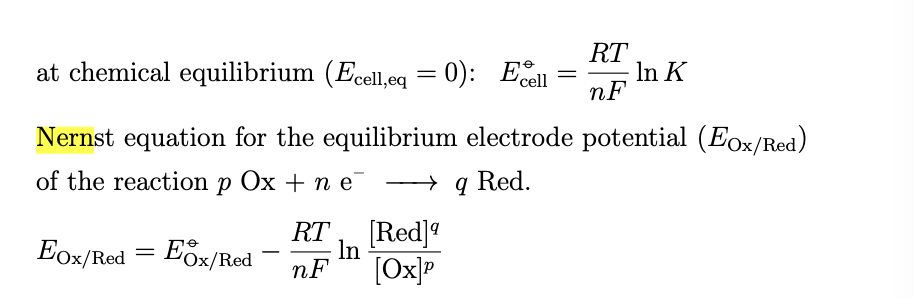
Nernst equation at chemical equilibrium
electrical conductance of a solution
When ions reach electrodes, they may not accumulate
often they accept or donate electrons at electrodes → continous current flows
migration rate depends on ionic mobility
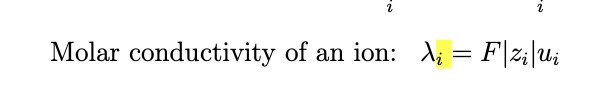
current from ion flow, each ion type i contributes
Ji is flux mol/m²/s
Zi is charge per mole with F being faradys constant
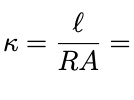
conductance
c= 1/R
conductivity removes cell geometry dependence
k=c x l/A
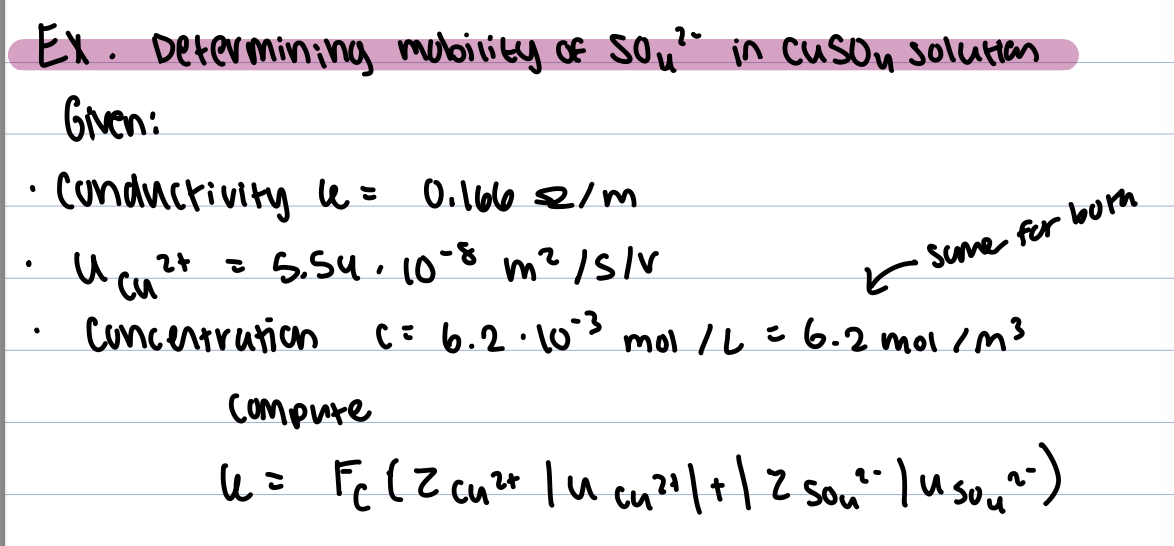

electrochemical reactions always involve
the movement of electrons from one chemical species to another
the species that loses electrons is the reducing agent and is oxidized
the species that gains electrons is the oxidizing agent and is reduced
redox reaction is a sum of two half reactions as a singel half reaction does not occur by itself
oxidation state
the oxidation state of an atom is the charge that the atom would have if all shared electrons were assigned to the atom which attracts them most strongly
rules to assign oxidation states
the oxidation state of an atom in an element is zero
the oxidation state of a monoatomic ion is equal to its charge
the sum of all the oxidation states of all atoms in a nuetral molecule is equal to zero and in an ion it is equal to the charge of the ion
the following atoms in compounds have the following oxidation states

Balancing redox reactions
write the two unbalanced half reactions
balance the elements other than hydrogen and oxygen for each half reaction
balance O by adding H2O or OH-
Balance h by adding H+
if the solution is acidic get rid of any OH- by adding H+ to both sides
if the solution is alkaline get rid of H+ by adding OH- to both sides, reduce any redundant water molecules
balance the charge by adding electrons
make the number of electron in each half reaction equal by multiplying one or both half reaction by a small integer number
add or subtract the two half reactions in such a way that the electrons cancel
get rid of any redundant terms
voltaic cell
a cell that generates electricity through a spontaneous redox reaction
electrolytic cell
consumes electrical current generated by an external source to drive a non spontaneous chemical reaction
each of the two half cells in an electrochemical cell consists of an electrode that is immersed in an electrolyte solution
the electrode where reduction takes place
is called the cathode, electrons enter the cell at the cathode and are taken up by the substance being reduced
the electrode where oxidation takes place
called the anode, electrons are given up by the substance being oxidized and leave the cell at the anode
to enable the cell to operate
the accumulation of charge in the solution must be nuetralised
this is achieved by joining the two half cells by a salt bridge which acts as a pathway that allows ions in the solution to flow from one half cell to the other
negative ions move from the cathodic to the anodic half cell nuetralising the accumulation of positive charge at the minus pole while positive ions in the salt bridge move in the opposite direction (nuetralising the accumulation of negative charge at the plus pole)
for some redox reactions
reactants and products of half reaction are present in the same phase, in that case a conductive surface is needed for electron transfer to take place
usually an invert electrode is used as the anode or cathode, this inert electrode is not oxidised or reduced, it merely accepts or donates electrons
shorthand notation for electrochemical cell
the vertical lines in this diagram represent the boundaries between the solid electrodes and the solution; the double vertical line that seperates the two half reactions denotes the salt bridge
anode on the left, cathode on the right with the charge change shown
electrical potential difference
between the two electrodes of the cell E cell= Er - El
the half cell that donates the electrons where oxidation takes place is drawn as the left cell
the way an overall reaction is written from the cell diagram is done by writing the two half reactions as reduction reactions and then doing right minus left equation
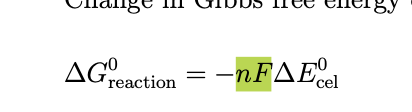
the drive to advance the reaction is
this reaction thus generates an electrical potential difference that pushes the electrons through the external electrical circuit
if we supply and Ecell of exactly the equation, the electron flow stops and in this situation the cell is in equilibrium
the reaction is not but the cell as a whole is
for this reason this balancing E cell is called the equilibrium cell potential
E cell eq is just another way of expressing how desperate the reaction is to proceed
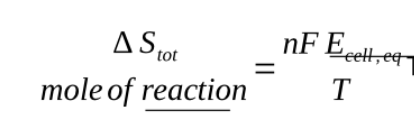
when Ecell is zero the reaction can go without any pushing back from the external circuit, the entropy production is then
to correct for non short circuited cells we just need to account for this pushing back effect
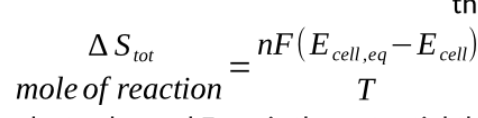
Ecell is the external potential difference, the voltage over the electrodes
Ecell eq is the potential that connects to the chemistry of the cell
the cell is used as a battery when E cell < E cell eq
when E cell > E cell eq the cell is charged
at equilbrium there is no entropy production so E cell = E cell eq
This equilibrium cell potential is also known as nernst potential at equilibrium there is no difference between Ecell and Ecell eq and therefore when it is indeed clear we are dealting with equilibrium we use Ecell = -gibbs energy/nF
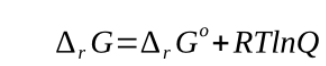

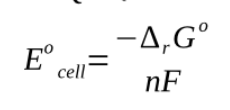

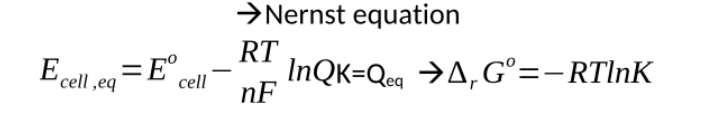
the equilibrium constant K relates to the concentration in the same way as does the reaction quotient with the only exception that all values pertain to chemical equilbrium
the same line of reasoning can be done for the nernst equation

SHE
standard hydrogen electrode
H+/H2 half cell
the potential of any other half cell relative to the standard hydrogen electrode can then be obtained by constructing a voltaic cell in which the half cell of interest is measured against SHE
SHE is always the left cell in the cell diagram
since we are dealing with the standard hydrogen electrode you can eliminate PH2 and {H+} from the equation because the value is 1, the corresponding nernst equation for the equilibrium electrode potential is then given by
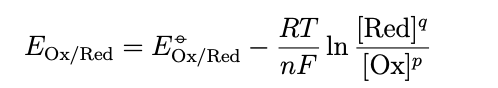
a species with a highly positive E standard eq has a high tendancy to attract electrons and is therefore a strong oxidising agent
a species with a highly negative E standard has a strong tedancy to repel electrons and is a strong reducing agent
the SHE serves as a common level to which all, what you can do to calculate equilbrium cell potentials from them
equilibrium electrode potentials are relative
start at the left cell decent to SHE level and climb to right cell level or do right minus left, standard cell potentials are just equilibrium cell potentials under standard conditions
therefore the standard cell potential of an electrochemical cell is found by subtracting the two standard electrode potentials
calculate equilibrium cell potential using the overall reaction equation or based on the equilibrium electrode potentials
To compute equilibrium cell potentials at arbitary concentrations use nernst equation ex
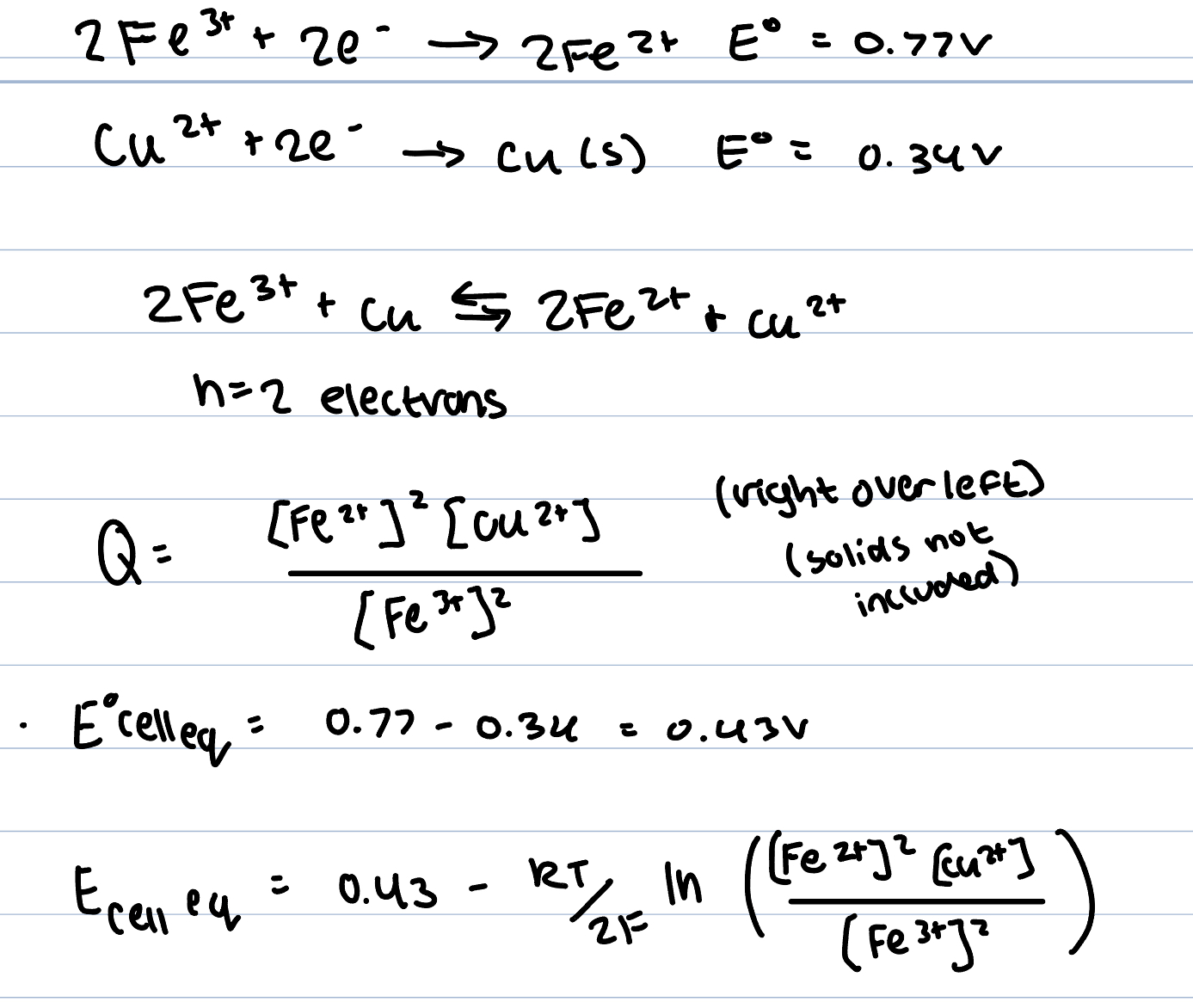
positive E cell eq indicates
The reaction is spontaneous to the right, the cell will be discharged
if set to a higher voltage (charging)
cell will be charged and electrons are forced to go from the RHS to LHS electrodes, reaction goes to the left
non spontaneous reaction
If Stot is negative
the reaction goes to the left and is not spontaneous, but if process naturally goes to the left like charging then Stot is actually positive and reaction will be spontaneous
If a reaction has a very large K
We can assume that the reaction goes to an end and stops working when the concentration of the reduced component Cu2+ equals zero
electrochemical cells can be used to measure concentrations
the potential of the indicator electrode is measured against a referance electrode of known composition
no electrical charge is allowed to flow so the referance electrode has a fixed equilibrium electrode potential
measure the potential of the indicator against the referance electrode so the indicator electrode is the half cell on the right in the cell diagram and the equilibrium electrode potential of the indicator electrode potential is found by adding the equilibrium electrode potential to the measured equilibrium cell potential
most used electrode
glass electrode used for measuring H+ concentrations
this electrode relies on membrane potential
when the glass electrode is placed in a solution of unknown pH, the measured electrical potential difference can be used to calculate the pH using the Nernst eqiation
In a potentiometric titration a redox reaction occurs between
the analyte and titrant
the equilbrium electrode potentials of the two half reactions that make up the redox reaction are mutually equal
the equilibrium electrode potential of the analyte solution is measured against a reference electrode
to measure the electrode potential we need a full electrochemical cell with two half cells
the second cell is some reference cell of constant composition
the two cells are connected by the salt bridge, since there flows no electrical current just to measure Ecell eq it is enough to know the potential of the reference electrode relative to SHE
as all components are in equilibrium throughout the titration both electrode potentials are equal to each other, they are the potential of the indicator electrode
in an electrolytic cell an external power source is used to drive
a non spontaneous redox reaction
by applying an external potential greater than the equilibrium potential, the reaction is forced to go in the other direction
in biological cells
glucose is oxidised in a number of steps, oxygen is the final electron acceptor
the energy that is released in this oxidation is used to produce ATP
If Ecell < Ecell eq
the term is positive → reaction proceeds forward (to the right)
If Ecell > E cell eq
reaction proceeds backwards (to the left) this is how recharging a battery forces reactions to reverse

atoms are made up of
a nucleus, consisting of positively charged protons and nuetral nuetrons, surrounded by a cloud of electrons
the nucleus occupies only a tiny part of the volume of the atom
atoms emission spectrum
discrete- only specific wavelengths are emitted
in bohrs model electrons can only occupy certain discrete orbits at specific distances from the nucleus
electrons can only gain and lose energy by jumping from one allowed orbit to another
while doing so they absorb or emit a quantum of electromagnetic radiation (a photon) with a wavelength that corresponds exactly to the energy difference between two orbits
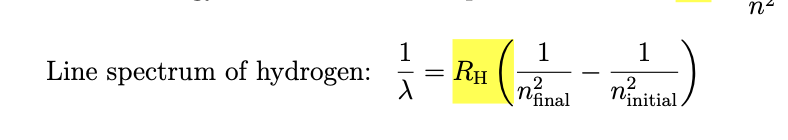
regular pattern in the series of lines in the hydrogen spectrum
RH is a constant
n is which orbital it falls to and starts from
n=2 is visible light
light is a type of
electromagnetic radiation, form of energy associated with oscillating electric and magnetic fields
travels through space at a speed c= 3 × 10^8 m/s
electromagnetic radiation has both wave and particle nature; the wave particle duality
wave nature is characterised by a
wavelength and frequency which are related troughly in above equation
wave nature manifests itself in diffraction and interference
the particle nature of light is characterised by a specific amount of energy carried in small light particles called photons
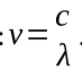
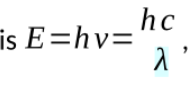
Energy of a photon
h is planck’s constant
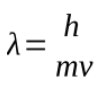
small particles also have wave length behaviour
their wavelength is given by de Broglies formula
h= plancks constant
m=mass
v=velocity
explains why electrons only have certain possible frequencies and energies
the wavelength of an object is inversely proportional to its mass, so heavy objects have wavelengths that are many magnitudes smaller than the object itself
A model for the structure of atoms in which the electrons were described as waves
made by combining Broglie’s wavelength with a standard wave equation
H stands for the Hamiltinoian operator
E stands for the actual energy level of the electron
Ψ is the wave function that represents the standing wave form of the electron
when the equation is analysed, a whole set of different solutions is found each solution consisting of a certain wave function Ψ that is called an orbital
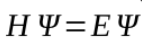
Each orbital represents a
possible state of the electron and to each orbital corresponds a particular value of E
the wave function Ψ corresponding to a certain orbital is a function of the position in space, as specified by three spatial coordinates x,y,z
we can only know the probability of an electron to be somewhere
the square of the wave function Ψ² expresses the probabilty density, the probability per unit volume for an electron to be at a certain location
solutions of the schrodiner equation for the hydrogen atom
characterised as three quantum numbers n,l, and m
n is an integer with values 1,2…. which determines the size of the orbitals
l runs from 0 to n-1 and determines the shape of the orbitals
m runs from l to -l and determines its orientation (for a given l value there are 2l+1 given m values)
letter notations are used to refer to orbitals with certain l numbers;
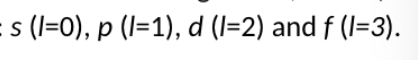

Energy values for hydrogen that come out of Schrodingers equation given by
RH is the rydberg constant
h is plancks constant
At infinite distance the energy is zero, this is the ionized state (the electron is removed)
the level with n=1 has the lowest energy, ground state
this is the most favourable state for the electron so the electron is most likely to be found here
when an atom absorbs energy
an electron in a lower energy orbital can be excited or promoted to an orbital with a higher energy
in this excited state the atom is unstable, and the electron quickly falls back to a lower energy orbital
as it does so it releases energy in the form of electromagnetic radiation
the amount of energy that is released is equal to the energy difference between the two orbitals
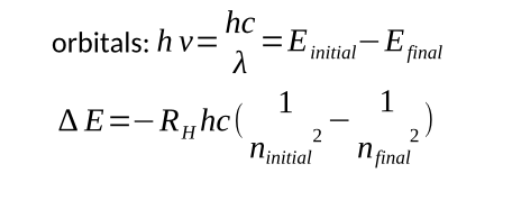
periodic table
elements exhibit a periodic recurrence of similair properties
the quantum mechanical model gives a natural explanation for this ordering scheme
orbitals for many electron atoms are similair to those of hydrogen atom
therefore we can use the s,p,d, and f orbitals also for multi electron atoms
however in order to see how multiple electrons occupy these orbitals we need two additional features 1) electron spin , a fundamental property of electrons that determines the number of electrons that can occupy an orbital
and 2) a more complex set of orbital energy levels
The three quantum number n, l, and mi describe the size, shape, and orientation of an atomic orbital
an electron can have two different spin states producing two oppositely directed magnetic moments
the new quantum number used to describe this is called the spin quantum number ms, can only have values +1/2 or -1/2 as a result each electron in an atom is described completely by four quantum numbers
No two electrons can have the same
four quantum numbers; the pauli exclusion principle
since electrons in the same orbital have the same values of n, l , and mi, this postulate says that they must have different values ms, since there are only two allowed values of ms each orbital can only hold two electrons and they must have opposite spins
For multi electron atoms the pattern of energy levels
is much more complex due to additional repulsion between electrons, to account for this reduced binding of the electrons we imagine that the electrons are held by an effective nuclear charge Zeff which is smaller than the real charge of the nucleus Z
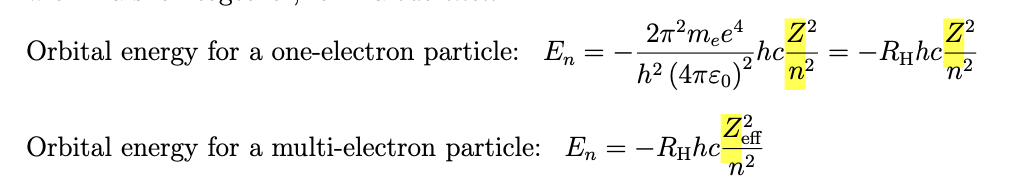
we can write the energy of an orbital as
the effective charge experienced by an electron depends on
the effect of nuclear charge- for one electron atoms the effective charge equals the real nuclear charfe (Zeff=z)
so when Zeff is twice as big, the orbital energy is two times lower
shielding
the effect of electron repulsion, for multielectron atoms, each electron not only feels the attractive positive charge of the nucleus but it also feels the repulsive negative charges of all the other electrons, these repulsive interactions between electrons increase the energy of the orbital
shielding of the nuclear charge by the inner electrons reduces the effective charge experienced by an electron in the outer shell
penetration
the effect of orbital shape; as the outer electron undergoes penetration into the region occupied by the inner electrons, it experuences a stronger attraction and therefore a lower energy
because electrons in s orbitals have a higher probability to be closer to the nucleus than p,d, or f orbitals (they penetrate more → higher Zeff) they have a lower energy

the aufbau principle can be built up as
we know that in the ground state electrons occupy the lowest energy orbitals available and that only two electrons with opposing spin are allowed per orbital, we can systematically build up the electron configuration for the elements
when two electrons occupy the same orbital
their spins must be opposite; they are paired
hunds rule
when orbitals of equal energy are available the electron configuration of lowest energy is the one with the maximum number of unpaired electrons with parallel spins
Electrons in different p orbitals are farther away from each other and therefore experience less repulsion → higher Zeff
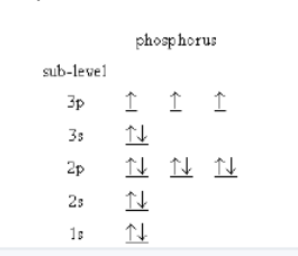
Elements in the same group and same period
have the same electron configuration in their outer shell
valence electrons
have the same amount of shells (inner shielding)
ionization energy trend (amount of energy to remove one mole of electrons from one mole of gaseous atoms )
increases when going left to right across a period due to same shielding and increasing nuclear charge by the added protons so Zeff will increase so the orbital energy becomes lower
going down a group the ionisation decreases as the outer electrons are farther away from the nucleus as n increases
electron affinity trends
the energy released when an additional electron is added to a gaseous atom in its ground state
for most elements the electron affinity is positive, because the extra electron is attracted by positive nuclear charge
but if you have a full shell it wont be positive and will actually require energy to occur
This increases across a period as same inner shielding and more willingness to gain electrons and decreases down a group as the electrons are farther away from the nucleus
The octet rule
Atoms will tend to share, gain or lose electrons to acquire a noble gas configuration
three types of bonds
1- ionic bonds; these are formed between atoms with large differences in their tendancies to lose or gain electrons, typically a metal (low ionisation energy) and a non metal (high electron affinity) electrons trasnfer from metal to non metal giving both a noble gas configuration
2- covalent bonds; electrons are shared by the two atoms, they have the same high ionization energy, the shared electrons interact with the nuclei of both atoms, and thereby lower the potential energy
3- metallic bonds; metals have a low ionisation energy, so they lose electrons easily, the outer electrons are bound so weakly that they do not stay with a single metal atom but move freely throughout the entire metal
bond polarity
when a covalent bond is formed between two different kinds of atoms, the electrons are usually not shared equally
one atom attracts them more strongly, the electron is partially transferred, such a bond is said to be polar (having a positive and negative pole)
by contrast bonds in which the electrons are equally shared are non polar
electronegativity
the ability of an atom to attract electrons in chemical bonds
the bond polarity depends on the difference in electronegativity between the atoms involved in the bond
dipole moment occur when
there is a seperation of positive and negative charge

with what difference in electronegativity are certain bonds formed

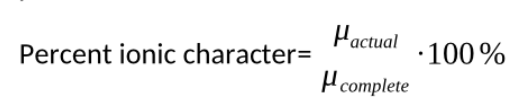
Percent ionic character
u actual is the actual dipole moment of a bond
u complete is if the electron transfers completely from one atom to the other (q=e)
for molecules with multiple bonds
the net dipole moment follows from adding the dipole vectors of each of the bonds, can cancel out if all moving in opposite directions and then the molecule is non polar with polar bonds
Lewis structures
write down the atom symbols according to presumed molecular layout, put the atom with the lowest ionisation energy in central position
determine the total number of valence electrons in the molecule, add or subtract extra electrons according to the charge of the molecule, this number is N
Determine the total number of valence electrons in the molecule if each atom would have a noble gas configuration, this number is M
A good guess for the number of bonding molecules in M-N, use these electrons to draw bonds between the atoms, each line represents two electrons
distribute the remaining electrons over the molecule as to satisfy the octet rule