Lecture 18 - Clinical Response of Normal Tissues II
1/79
Earn XP
Description and Tags
ONCOL 335. Radiobiology. University of Alberta
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
80 Terms
Why is the inherent radioresponsiveness of normal tissues difficult to measure in humans?
very few human cells lines can be grown in vitro
what types of cells could be used for a clonogenic survival assay to attempt to measure healthy cell radioresponsiveness?
fibroblasts, lymphoblasts and potentially keratinocytes
what correlation is there between the radioresistance of fibroblasts and radioresponsiveness of normal cells?
weak correlation
how do we know radioresponsiveness is host-specific?
we have evidence for similar sensitivity to the same complication in different sites of the same individual
how do the different tissue’s stem cells radiosensitivity compare?
they are all relatively in the same region with exception to bone marrow being far more sensitive to radiation
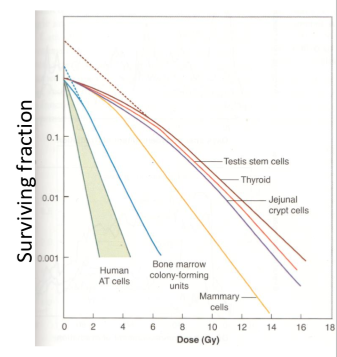
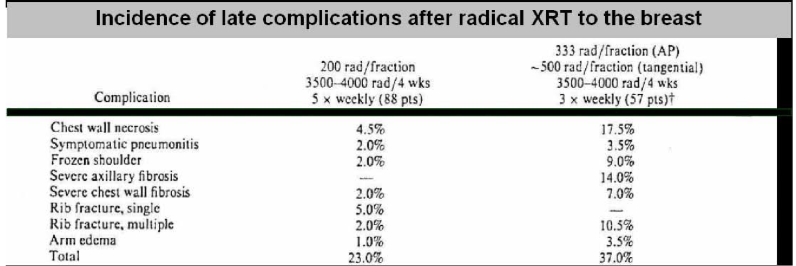
what does this research tell us?
there is clinical evidence for a difference between early and late effects in response to alteration of fraction size for the same total dose and overall treatment time
3.33/5 Gy per fraction caused significant reduction in late effects than 2 Gy fractions
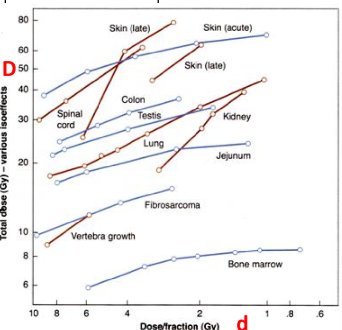
What are isoeffect curves?
plots of combinations of total dose (D) and dose per fraction to which give the same degree of tissue injury
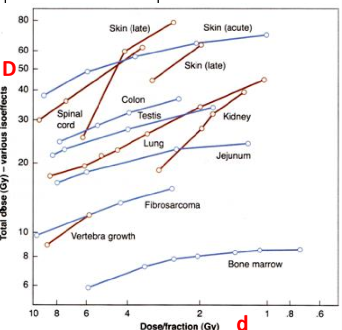
do iso-effect curves describe late effects to have steep slopes or less-steep slopes
steeper slopes
early effects have less steep slopes
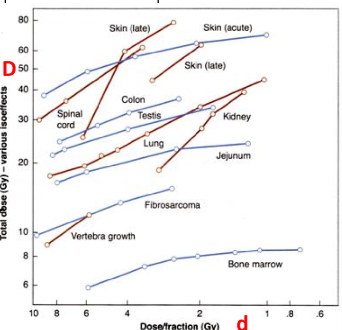
what does the increased slopes of late effects tell us in regards to fractionation
late effects are spared more by fractionation
you need a larger total dose to reach late reactions if you fractionate
review: what are the two components of the LQ model?
alpha component of cell kill is proportional to dose
beta component of cell kill is proportion to dose²
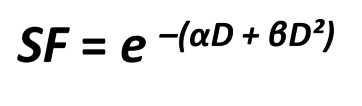
what is the alpha/beta ratio?
the dose where the linear component of kill equals the quadratic component of kill
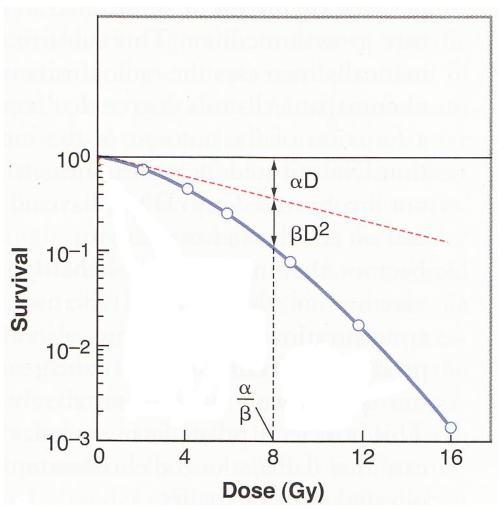
do late effects on normal healthy tissues have a high or low alpha/beta value?
normal tissues late effects typically have a low alpha/beta
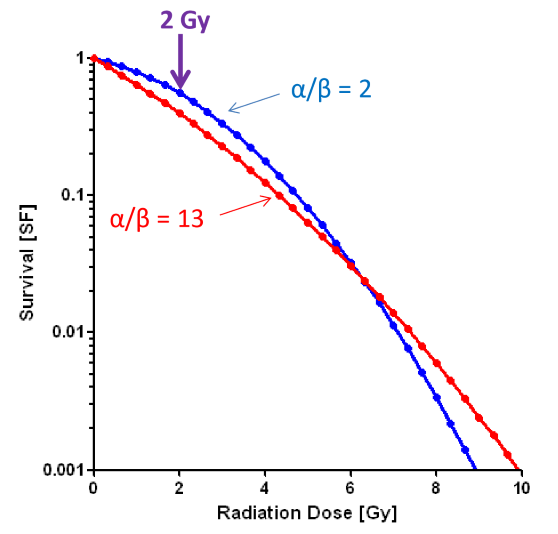
do early effects on normal healthy tissues have a high or low alpha/beta value?
normal tissue early effects have a high alpha/beta
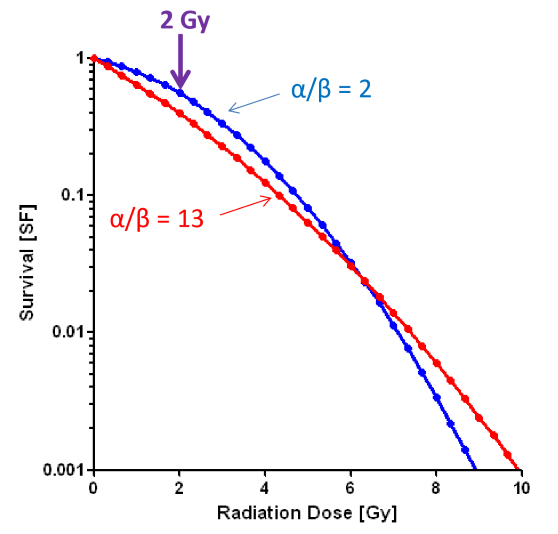
since tumors have a high alpha/beta ratio, what does this tell us about the side effects of treatment we get in healthy tissues
we will get early effects (in addition to tumor cell kill)
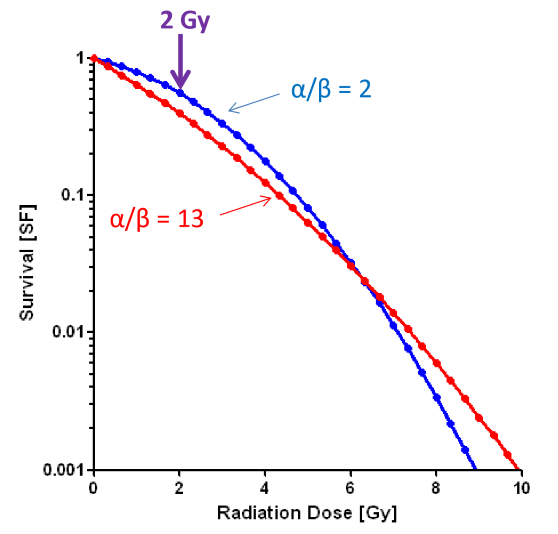
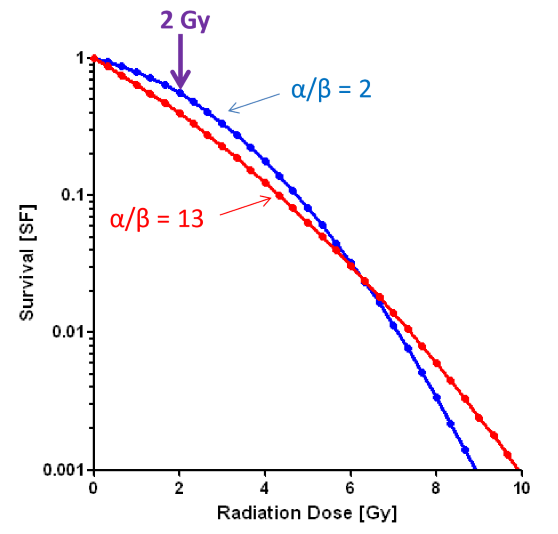
what effect does fractionation have on the LQ model?
we can now see a difference between the blue and the red line
this is evidence that fractionation will effect high alpha/beta cells (like tumors and early effects) more than low alpha/beta cells (normal tissue long term effects)
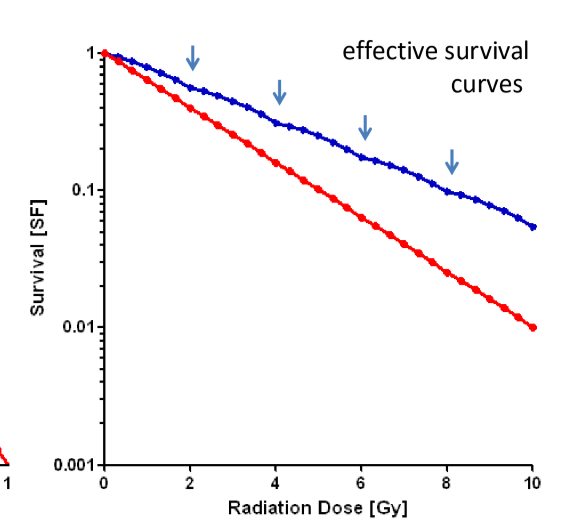
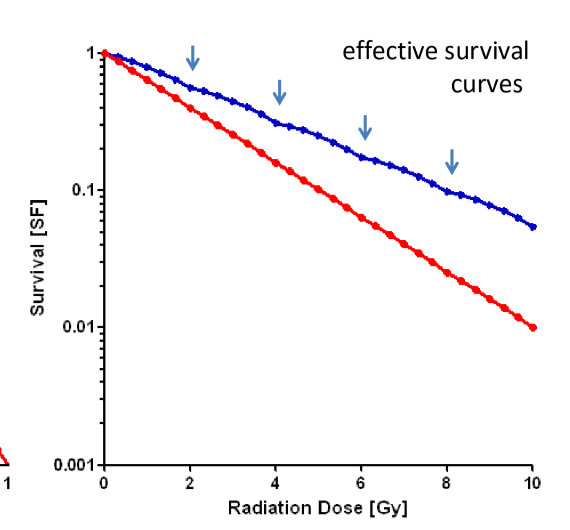
how much dose is needed for low alpha/beta cells to receive the same biological effect as high alpha/beta cells?
more dose is needed to have the SF of blue line to be equal to SF of red line
thus normal tissue is more spared with fractionation
why can’t the LQ model be verified directly?
there is no single-dose survival curve data for cells that come from late-responding normal tissues
this is why single-dose survival curves are calculated using inverse process method based on fractionation data
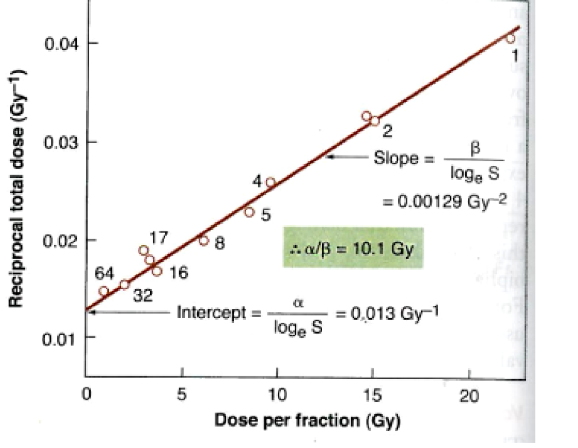
what is the inverse process method for single dose survival curves?
we reciprocate the total dose and then find alpha/beta with information from the slope and the intercept
do early reactions have low or high alpha/beta
high
this is why we see these side effects when we fractionate
have similar alpha/beta to tumors
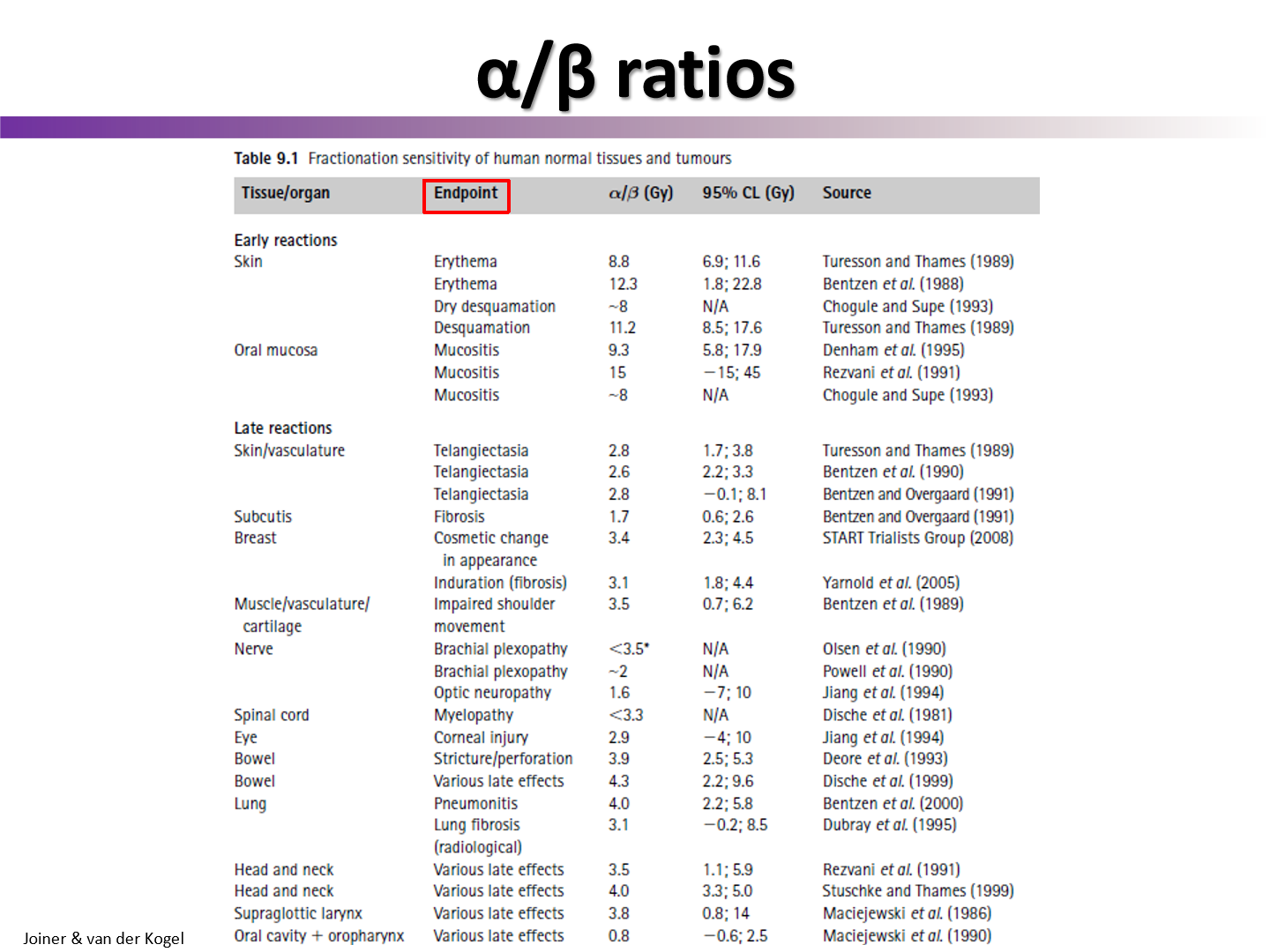
do late reactions have high or low alpha/beta
low
this is why we spare these effects when we fractionate
have similar alpha/beta to healthy tissue
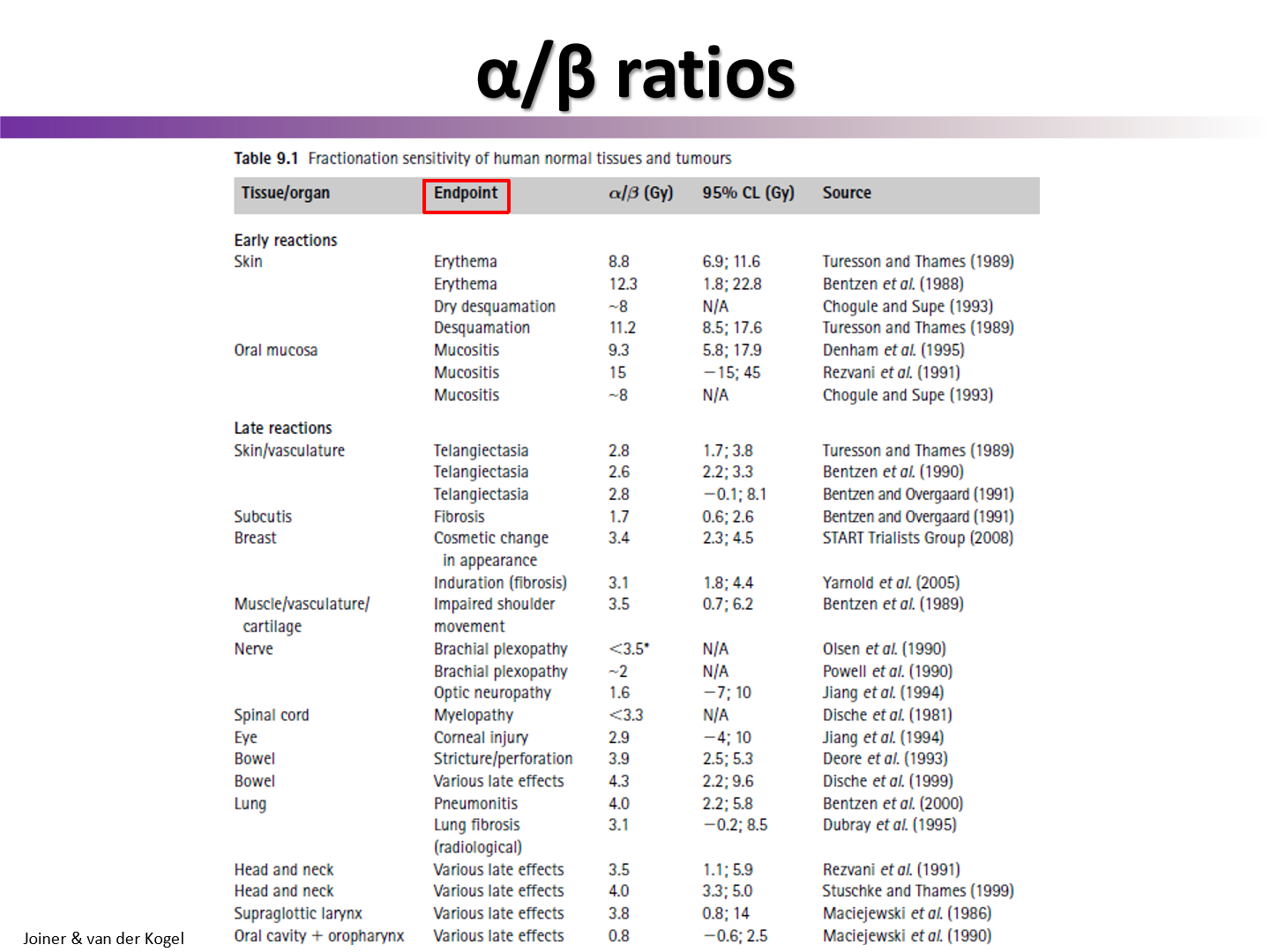
what is the only tumor type that has a low alpha over beta
prostate cancer
since prostate cancer has a low alpha/beta, what type of fractionation plan should be used?
hypofractionation
Although not proven, what is a plausible explanation as to why there is a difference in alpha/beta ratios between late response vs. early responses (and tumor responses)?
differences in repair of potentially lethal damage between early and late responding normal tissues may be a factor
why might repair of potentially lethal damage be greater in late response normal tissues than for early response normal tissue?
in slow proliferating cells (which are typically late-responding tissues), repair of damage should continue for longer times, leading to more repair for the same amount of initial damage
potentially lethal damage definition
damage that could cause death, but is modified by post-irradiation conditions
when is potentially lethal damage repaired?
during the interval between treatment and assay
how does the repair of fast proliferating cells (high alpha/beta) compare to slow prolifereating cells (low alpha/beta)?
cells that proliferate fast will have a dip in their recovery factor since the surviving cells moved on to a more sensitive phase
slower dividing cells don’t get this dip
essentially cells resensitize if they move into a more radiosensitive part of the cell cycle
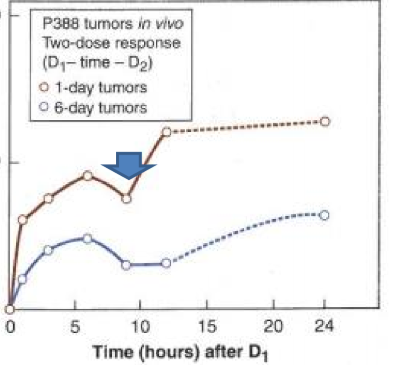
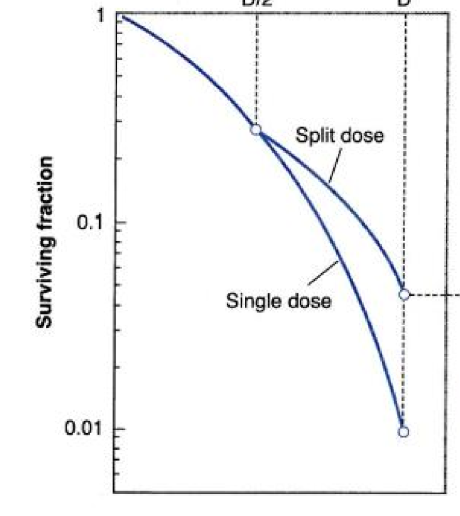
what happens to repair when we fractionate?
we get a shoulder due to the cells getting time to repair some of their damage
5 R’s of radiobiology
radiosensitivity
repair
reoxygenation
Reassortment
redistribution of cells in cell cycle
repopulation
regeneration of cells
what is another plausible explanation as to why there is a difference in alpha/beta ratios between late response vs. early responses (and tumor responses)?
tissues are heterogenous with their cells and what phase of the cell cycle they are in
typically late responding tissues will all be in one phase of the cell cycle
while early responding tissues will all be in another phase of the cell cycle
this could result in an overestimate of the a/B ratio in fractionation experiments
what is one more explanation as to why there is a difference in alpha/beta ratios between late response vs. early responses (and tumor responses)?
there is more involved in normal tissue complictation than cell death
ex: inflammation may play a role in cell response
what are the three explanations as to why there is a difference in alpha/beta ratios in late response and early response healthy tissues?
different repair capacities of potentially lethal damage
cell cycle heterogeneity in tissues increases a/B ratio
there is more to non-tumor complications than just cell death
differences between normal wound healing and radiation induced wound
In normal wound healing we get collagen synthesis and degradation but for radiation we get collagen depostion
this decreases functionality of fibroblasts
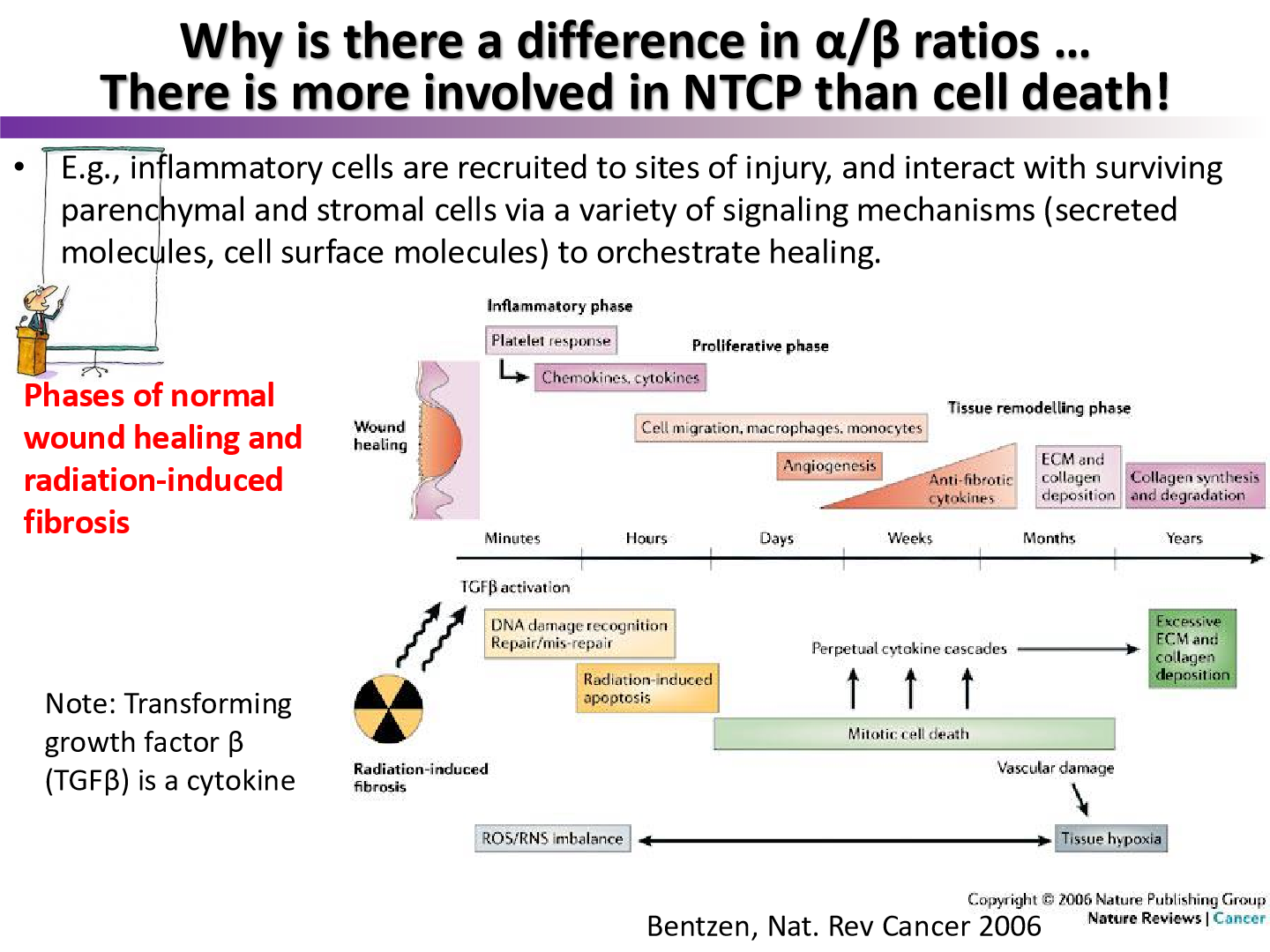
what cytokine plays an important role in radiation induced fibrosis, resulting in the difference between normal wound healing and radiation
TGF-beta
what is radiation induced fibrosis in fibroblasts?
after radiation stress, fibroblasts often go into senescence
TGF-beta upregulated = fibrosis
p21 upregulated = cell cycle inhibtion = senescence
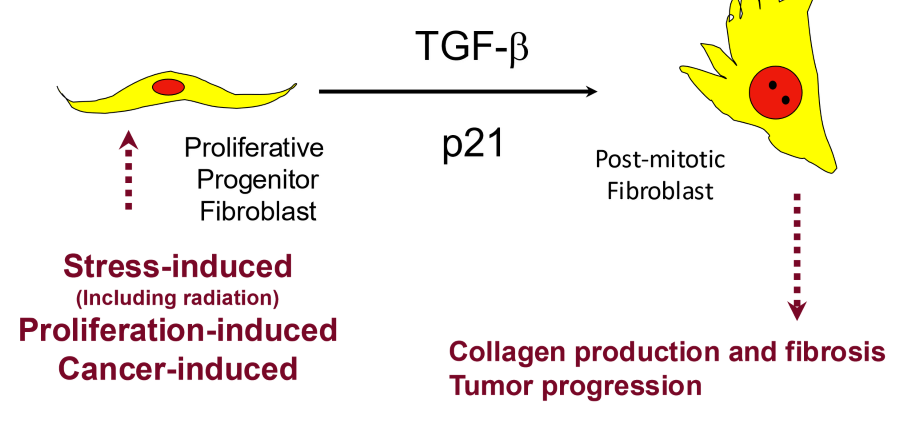
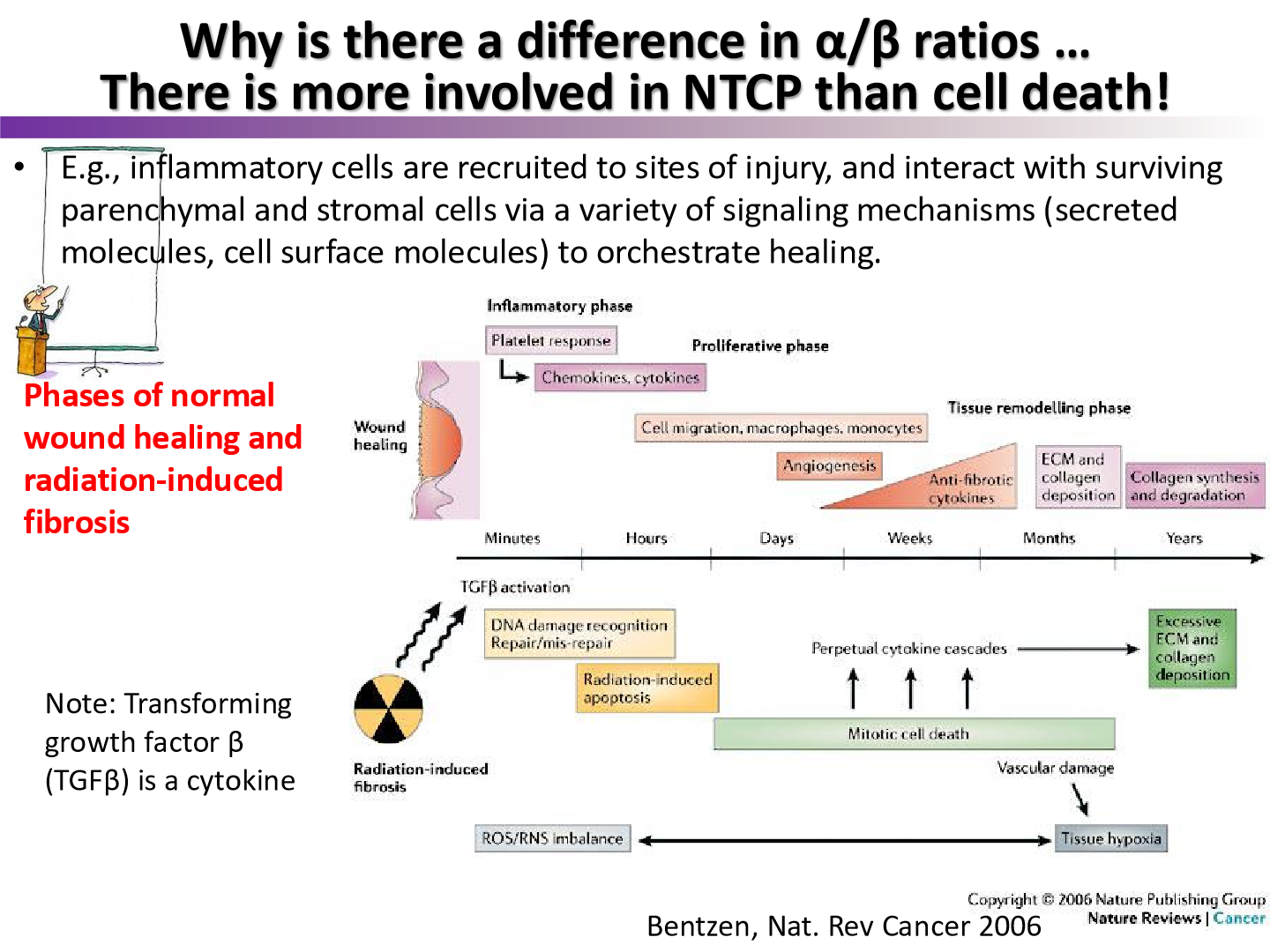
what was the model of normal wound healing in this figure called?
the Bentzen model
what does the Bentzen model say about normal wound healing and radiation wound healing
normal wound healing is precisely orchestrated
radiation wound healing has distinct processes that may interfere with normal control of wound healing
according to the Bentzen model, what distinct processes occur in radiation wounds?
excessive deposition of ECM and collagen which lead to radiation fibrosis
why is it important to consider physiological response to radiation or normal injury?
may impact how other tissues respond to injury
for example: kidney radiation may increase blood pressure via angiotensin activation
increased blood pressure effects how other tissues react to injury
what type of exposure are most of the experiments discussed up to this point deal with
total body exposure
we have usually irradiated complete organ of mouse or complete tissue
what does the volume effect attempt to answer
what happens when only part of an organ/tissue is irradiated, and not the entire structure
what plays a big roll in the volume effect?
tissue architecture
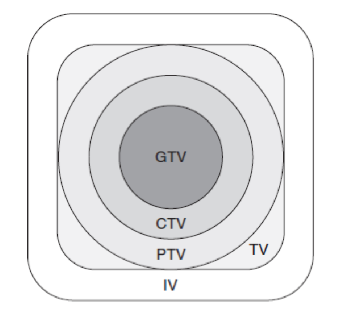
radiation volumes: GTV
gross tumor volume
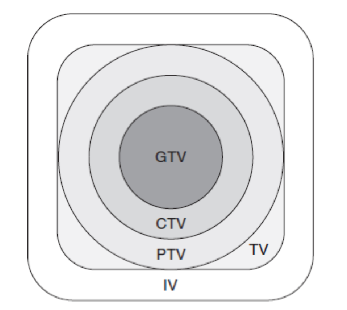
CTV
clinical target volume
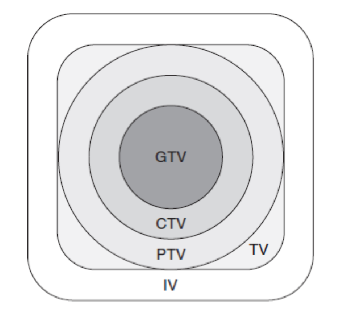
PTV
planning target volume
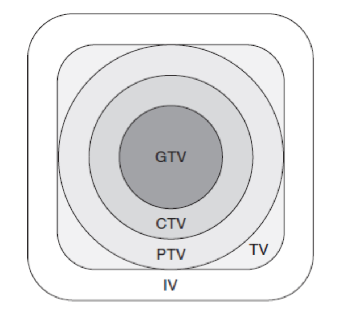
TV
treatment volume
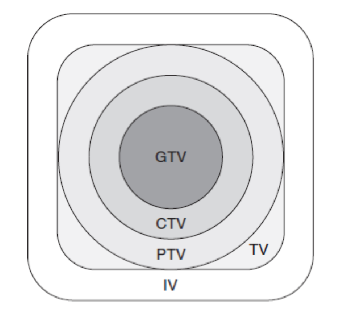
IV
irradiated volume
what does the target/critical volume model assume tissues are composed of
functional subunits (FSUs)
within this model, what are the two types of functional subunits?
structural
functional
structural FSUs
each subunit is divided and independent
there is a physcial barrier preventing surviving cells from repopulating adjacent FSUs
examples of structural FSUs
intestinal crypts
liver lobes
nephrons of kidney
functional FSUs
the largest volume that can be repopulated by a single surviving cell
examples of Functional FSUs
spinal cord, optic nerve
how is the function of an FSU lost?
FSU must lose all of it’s subunits
crucial variables in losing FSU function
organization of FSUs (parallel or serial)
only this one is important to remember
also
number of cells per FSU
more stem cells = trickier to kill them all
number of FSUs per organ
reserve capacity of tissue
how many FSUs need to be destroyed to cause complication
Parallel vs. serial organization of FSUs (think like circuits)
Parallel FSUs
FSUs funciton independently and organ function is sum of function of FSUs
Serial Organization
each FSU is a critical element
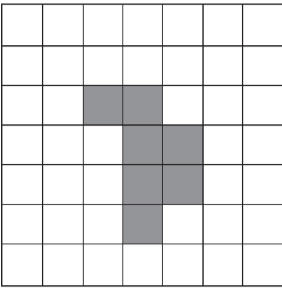
What causes damage in parallel FSUs
a critical number of functional subunits must be damaged before a clinical response manifests
this is called critical volume (mu)
example of critical volume (mu) for parallel FSUs
in kidney, ~90% of nephrons need to die to result in damage manifestation
therefore mu = 0.9
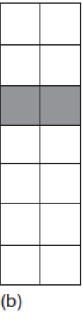
What causes damage in serial FSUs
failure of one FSU results in loss of function for the entire organ
one FSU is called a critical element
example of a critical element for serial FSU
damage to one lower motor neuron can cause flaccid paralysis
will serial FSU organ/tissues be more sensitive to whole organ radiation or partial organ radiation?
partial organ radiation
all we need is one FSU to be damaged to cause organ failure
will parallel FSU organs/tissues be more sensitive to whole organ radiation or partial organ radiation
whole organ radiation
what are serial and parallel FSUs an important example of
how important tissue architecture plays a role on the critical volume theory
why is the curve so steep for the spinal cord graph?
due serial FSU organization
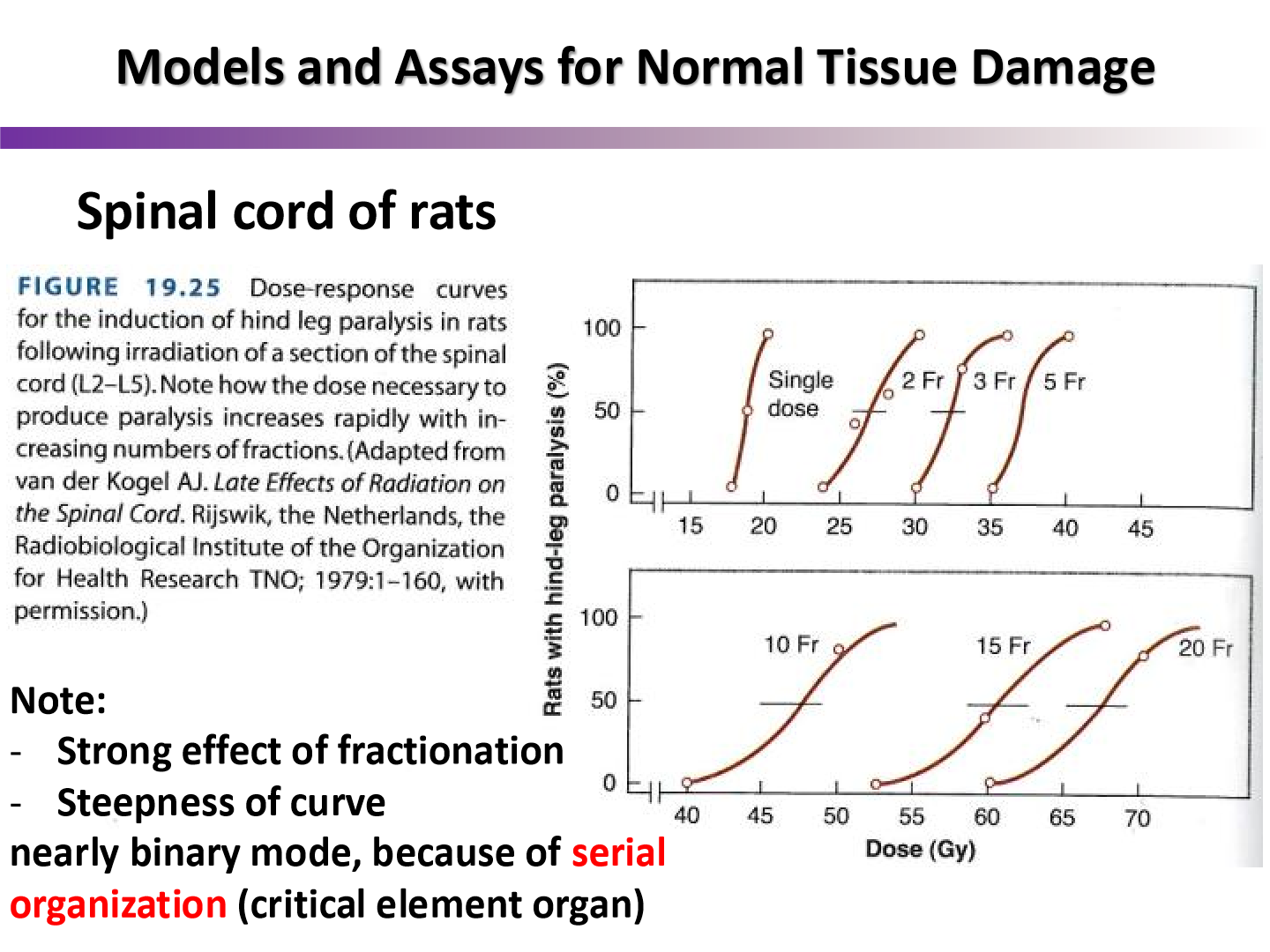
what type of curve do parallel architecture organs follow?
threshold curve
more than 70% of FSUs must be destroyed to cause complication
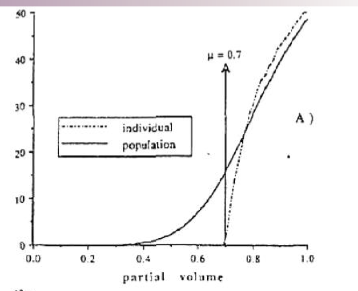
what type of curve do serial architecture organs follow?
linear curve
one FSU being destroyed causes complication
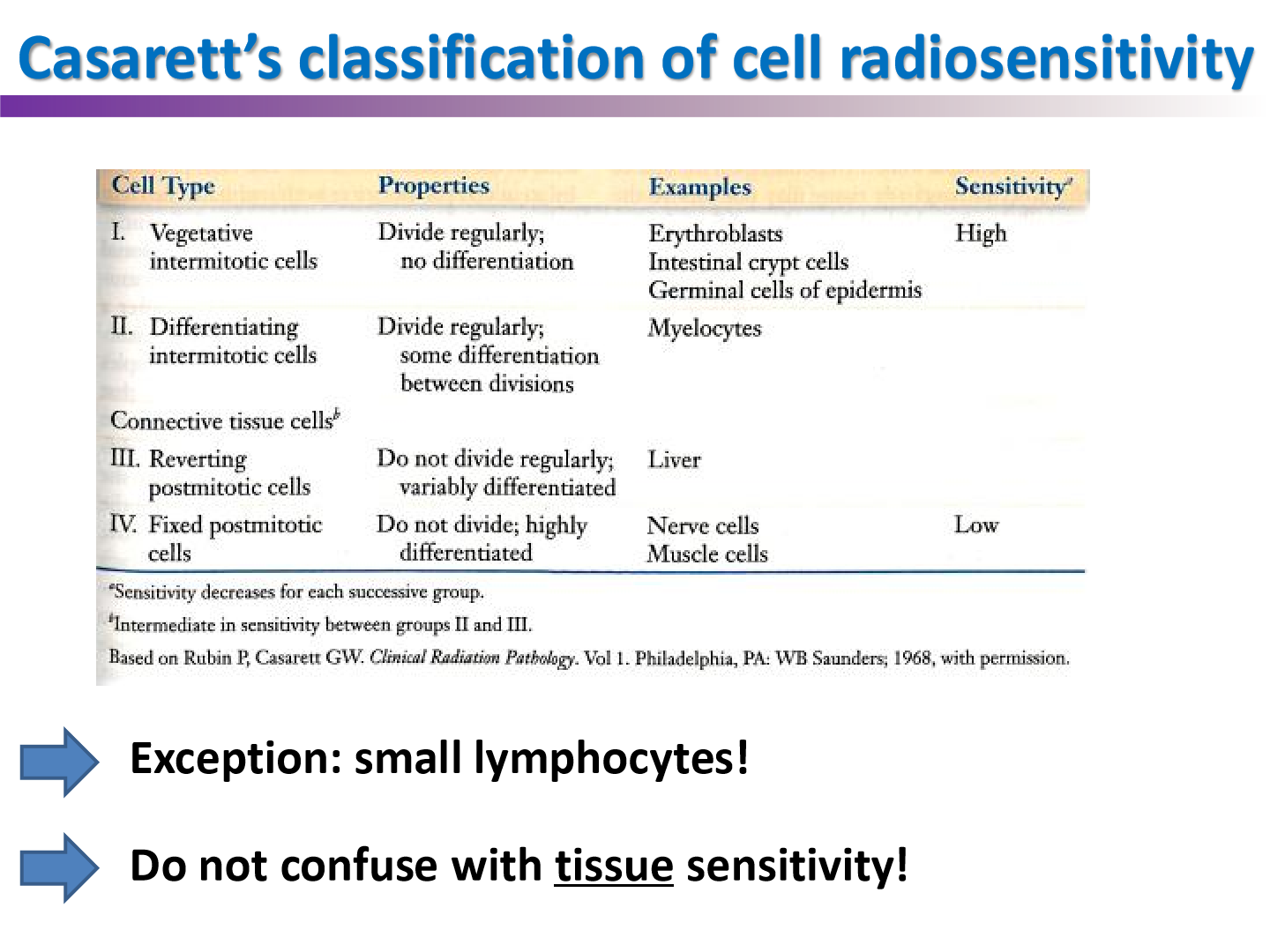
casarett’s classification of cell radiosensitivity
examples of parallel homogenous organs
lung, liver, kidney
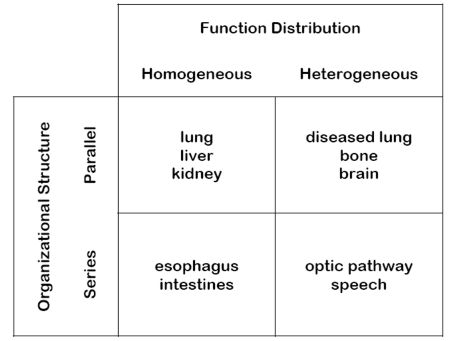
examples of parallel heterogenous organs
disease lung, bone, brain
examples of serial homogenous tissues
esophagus, intestines
examples of serial heterogenous tissues
optic pathway, speech
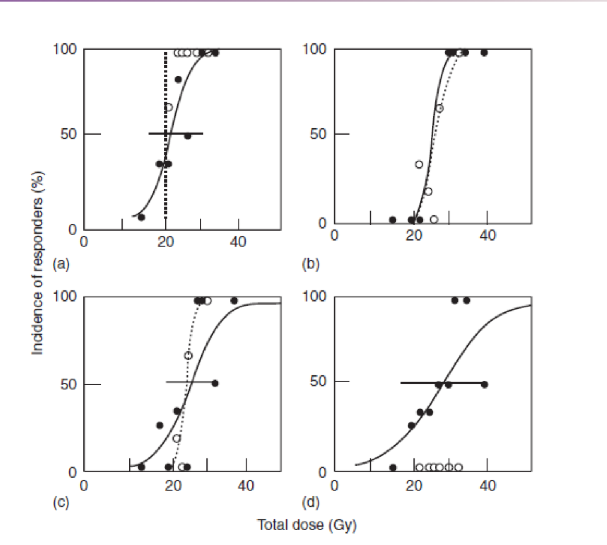
what do these graphs tell us about whole lung and half-lung irradiation
there is not much of a difference in terms of dose vs. damage ( in top row), but there is significant difference in dose vs. function
fibrosis will happen no matter how much of lung is irradiated, but decrease in tissue function only occurs if you irradiate whole lung
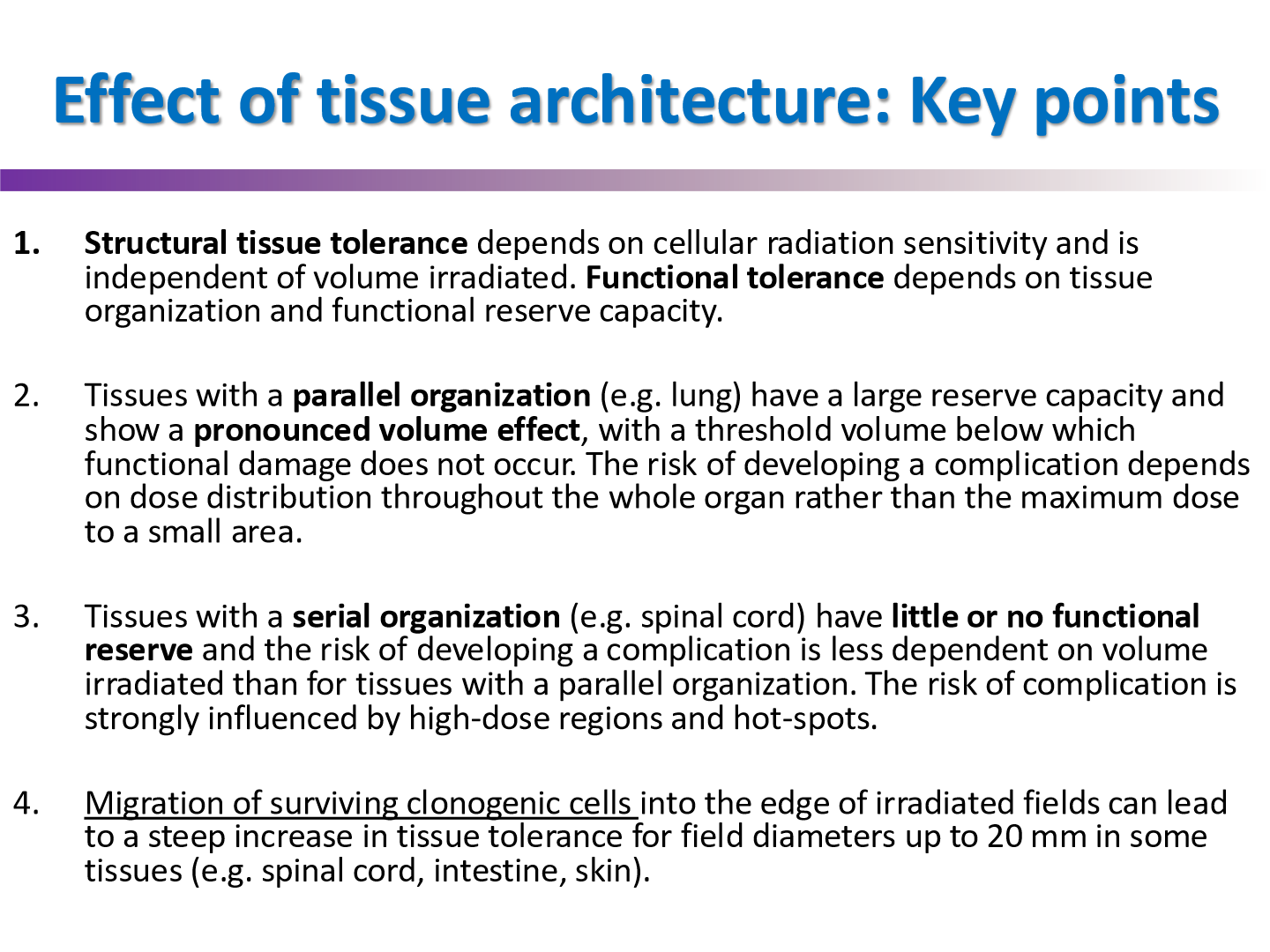
effect of tissue architecture 4 key points
Functional Subunit
largest tissue volume or unit of cells that can be regenerated from a single surviving clonogenic cell
if the volume of the tissue damage is less than critical damage, will complications occur in parallel organs?
no
in organs with serial organization, each FSU is _____
essential (a critical element)
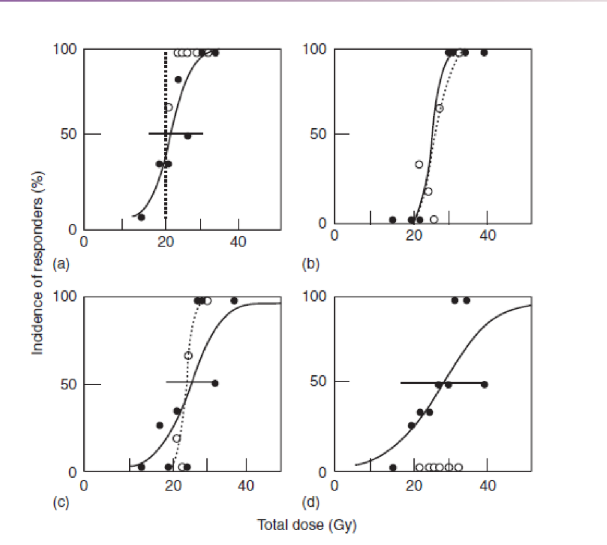
why is inter-individual variability to radiation important for the clinic
complications of radiation will vary individual to individual
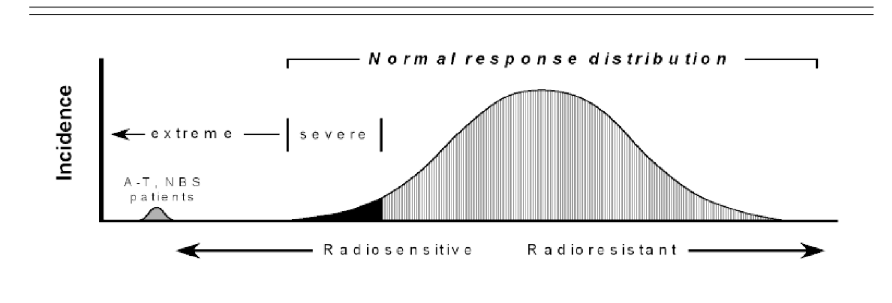
people with what mutations are extremely radiosensitive?
People missing ATM or NBS genes
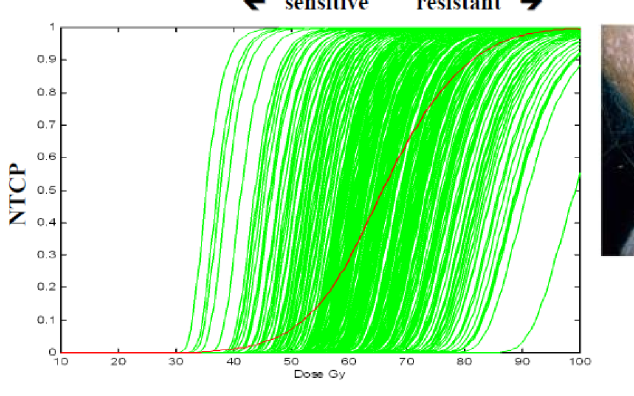
what assays can provide an idea of radiosensitivty for the patient (although not feasible)
clonogenic survival assays
what may be the best way to determine radiosensitivity of patients
genetic screenings to detect for certain genes that make you radiosensitive
but we don’t have too many biomarkers aside from ATM and NBS