3.2.5.6 catalysts
1/45
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
46 Terms
why can transition metals be used as catalysts?
as they have variable oxidation states
what is a catalyst?
a substance that increases the rate of reaction but isnt used up in the reaction
how does a catalyst work?
they provide an alternative route with a lower activation energy
how does a catalyst affect a reaction at equilibrium?
a catalyst has no effect on the position of equilibrium as it increases the rate of the forward + backwards reactions equally
it only decreases the time taken to reach equilibrium
outline the example of catalytic converters in a car exhaust
platinum, palladium or rhodium are sprayed over a ceramic honeycomb structure to limit costs + maximise surface area
what are the two types of catalysts?
heterogeneous catalysts
homogeneous catalysts
outline what heterogeneous catalysts are
these are catalysts which are in a different phase to the reactants
the catalyst is usually a solid + the reaction takes place on the surface
outline 2 examples of heterogeneous catalysts
haber process - industrial production of ammonia
contact process - for making sulfuric acid
explain how heterogeneous catalysts work?
at least one of the reactants is adsorbed onto the surface (ie forms bonds to the atoms in the solid surface)
adsorption is to go onto something, but absorbed is to go into something
the places on the surface where molecules are adsorbed are called active sites
in an effective catalyst, the molecules can move about the surface, bonding to different active sites
the adsorption of reactants onto the surface can result in an increases reaction in a number of ways:
adsorption onto the surface effectively concentrates the reactants ie brings them closer together than in the gas phase, so increasing the likelihood of collision
it may weaken some of the bonds in the molecules, making reaction easier
it may position the molecule in a favourable orientation for reaction
what happens if adsorption is too weak?
then not many molecules will be adsorbed so the catalyst will have very little effect
what happens if adsorption is too strong?
then molecules will not be able to move around the active sites + so be less likely to meet another reactant + so be less likely to react (also any product will tend to remain adsorbed on the surface)
why does the reaction occur with a lower activation energy?
as bonds are weakened or new bonds are made between reactants held close together
the products are then …..
desorbed (leave the surface)
why is maximising surface area important?
many surface catalysts are very expensive + so maximising SA has important cost savings
outline how the surface area is maximised
by using a very thin coating of the catalyst on some type of support medium
(a support is required as the layer is too thin to support itself, often a ceramic '“honeycomb” structure is used as support)
for the haber process, give the word, symbol equation + the catalyst

give the overall word, symbol + catalyst of the contact process
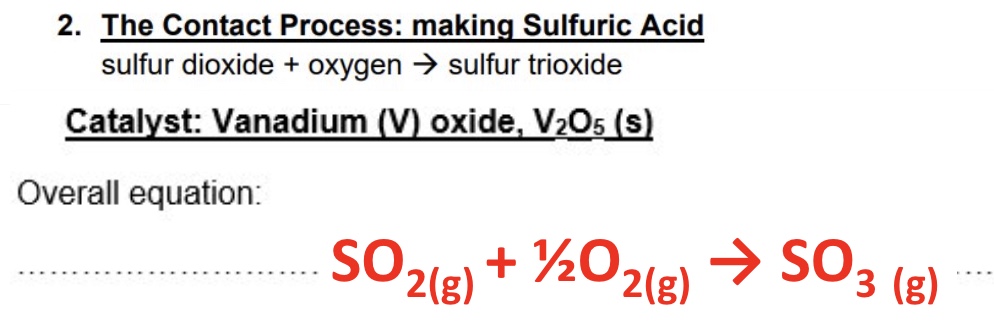
give the equations of step 1 and step 2 of the contact process
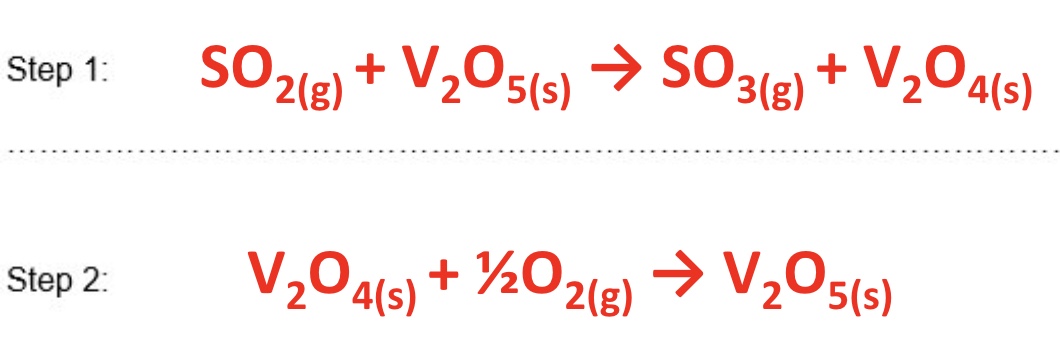
what are some heterogeneous catalysts prone to?
to poisoning
what is catalyst poisoning?
where other substances (impurities) adsorb strongly to the surface, blocking the active sites
outline the problems with catalyst poisoning
this lowers the efficiency of the catalyst or makes it totally ineffective depending on the extent of the poisoning
these poisons are extremely difficult to remove + the catalyst is ruined, which can be very costly (particularly with expensive catalysts)
outline one example of poisoning using catalytic converters
lead poisoning of catalytic converters in cars
both the Rh and Pt catalysts are poisoned by lead (from leaded petrol) + are very expensive to replace
outline one example of poisoning using the haber process
sulphur poisoning in the haber process
the hydrogen is obtained from natural gas which is contaminated by sulfur which if not removed will poison the Fe catalyst (sulfur is added to natural gas to give it an odour, so leaks can be smelt)
what are homogeneous catalysts?
these are catalysts which are in the same phase as the reactants
where do most reactions involving homogeneous catalysts take place?
in solution (aq)
what does the alternative reaction pathway, with homogeneous catalysts, often involve?
often involves the formation of an intermediate - with a different oxidation state
give 2 examples of homogeneous catalytic processes
reaction between iodide ions (I⁻) + persulfate ions (S₂O₈²⁻)
reaction between ethanedioate ions (C₂O₄²⁻) + manganate ions (VII) ions (MnO₄⁻)
for the reaction between iodide ions (I⁻) + persulfate ions (S₂O₈²⁻), give the catalysts + overall equation

why does the reaction between iodide ions (I⁻) + persulfate ions (S₂O₈²⁻) have a high activation energy?
both reactants are negatively charged and so will repel each other

for this reaction, give the reducing + oxidising agent


for this reaction, give the 2 half equations for the oxidising and reducing agent
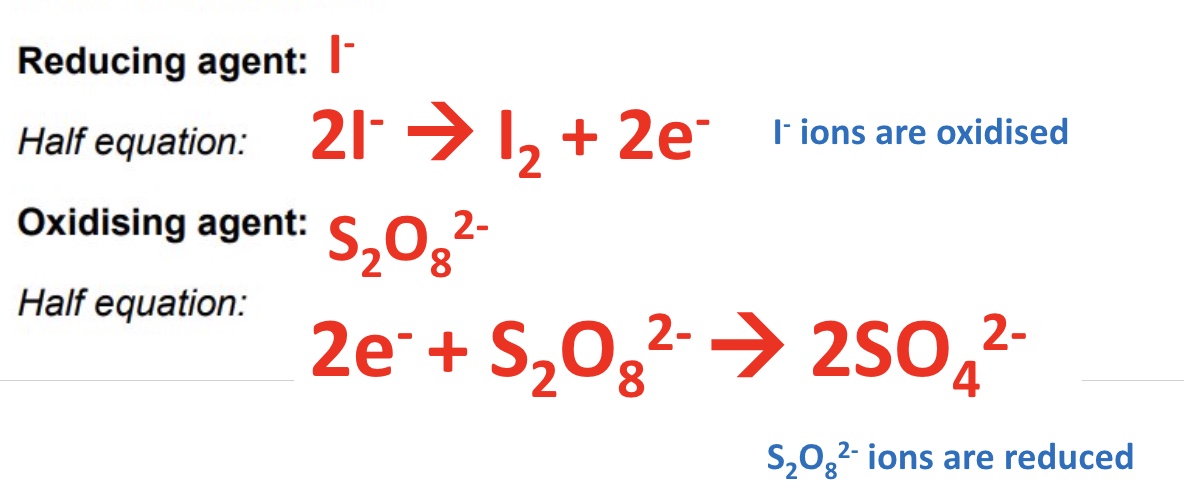

Fe²⁺ (aq) ions are added as a catalyst, why will the reaction be faster with these ions?
opposite charges on ions attract, activation energy is lowered
write two half equations to show Fe²⁺ (aq) changing oxidation state
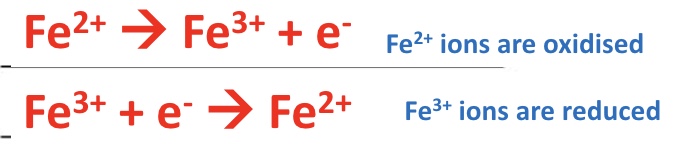
give the reactions for step 1 and step 2 for the catalysed reaction (ie with Fe²⁺ or Fe³⁺)


for this reaction,
a. give the overall equation
b. catalyst
c. where ethanedioate ions come from and the structure of them
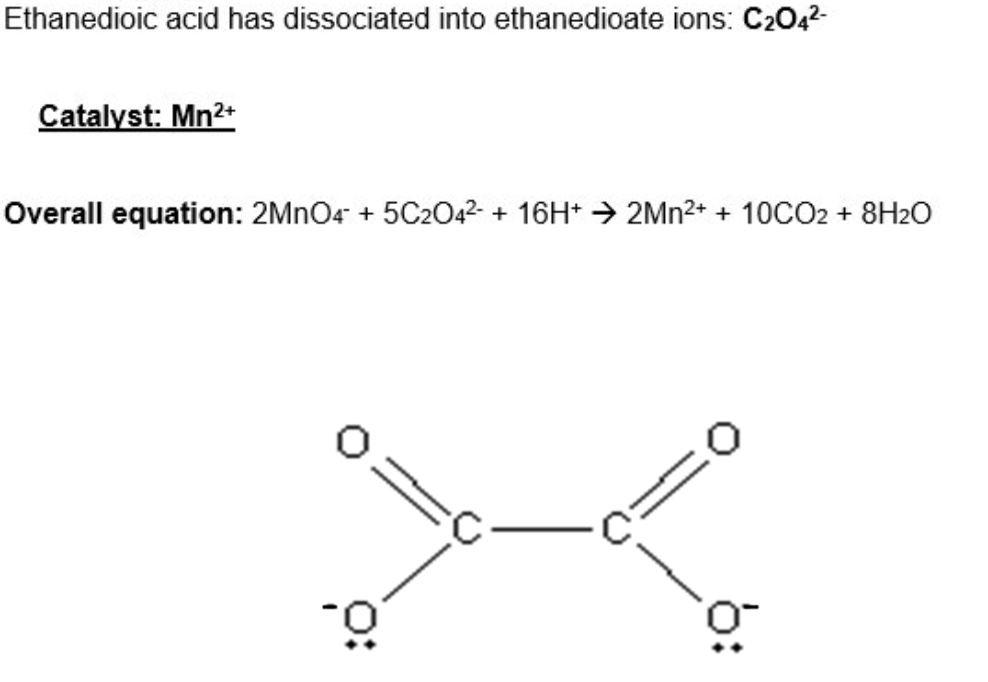

what is this reaction an example of?
autocatalysis
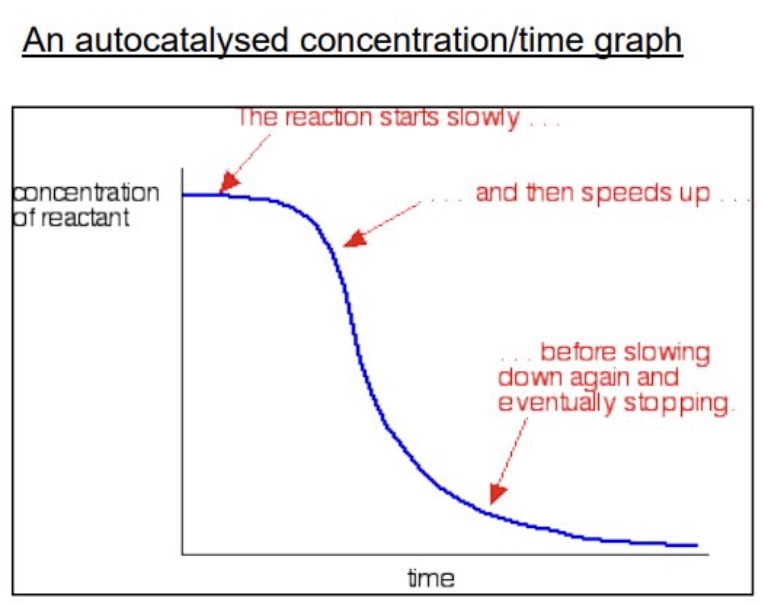
what is autocatalysis?
it occurs when one of the products from a reaction is a catalyst for the reaction
outline changes for the rate of reaction for ones with autocatalysis
reaction will start out slowly
as concentration of the catalyst product increases, the rate will also increase

for this reaction give the 2 half equations that show oxidation and reduction
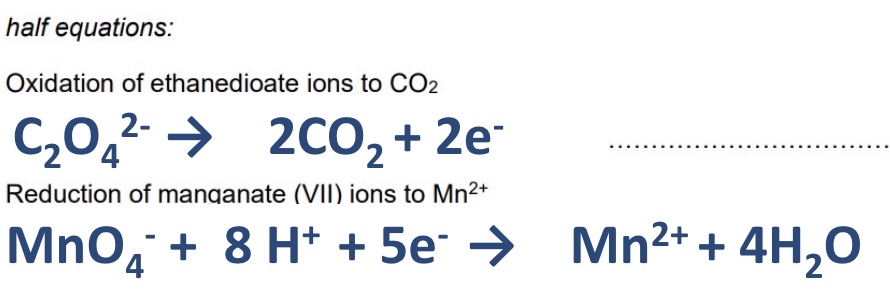

write two half equations to show the Mn²⁺ (aq) catalyst changing oxidation state
Mn²⁺ → Mn³⁺ + e⁻ (oxidation)
Mn³⁺ + e⁻ → Mn²⁺ (reduction)

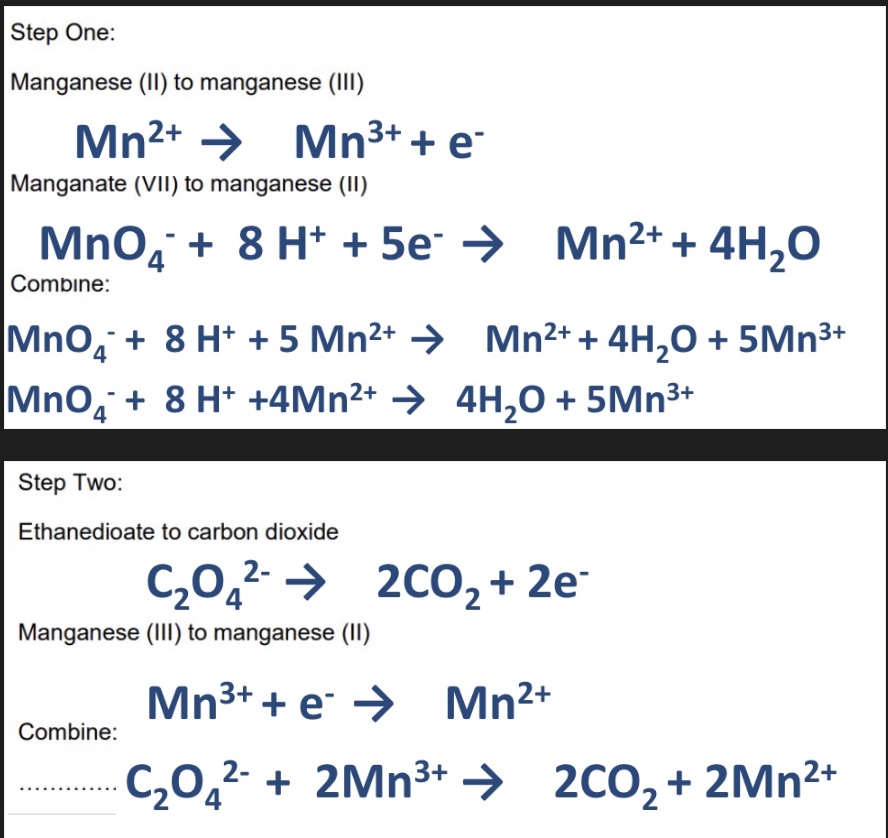
for this reaction, combine the 4 half equations to give 2 equations for the 2 steps
use loop method to figure out the equations

why is this reaction very slow in the absence of a catalyst?
because it involves the reaction of two negative ions, which repel each other
however the reaction is catalysed by Mn²⁺, why can this act as a catalyst?
because it has variable oxidation states Mn²⁺ and Mn³⁺
because the catalyst is a product of the reaction, what does this mean?
the reaction is slow until some Mn²⁺ is formed but then it speeds up as catalyst is formed
draw a normal concentration/time graph
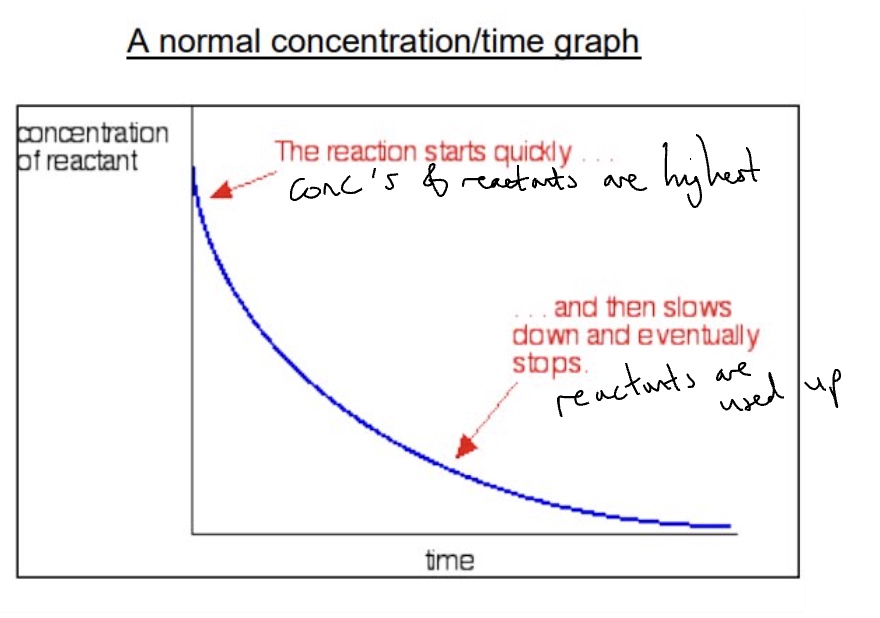
draw + explain a autocatalysed concentration/time graph
reaction starts slowly because negative ions repel + no catalyst has been produced yet
rate/slope increases as catalyst forms
rate decreases as reactants are being used up