Chemistry-VSEPR Theory
0.0(0)
Card Sorting
1/11
Earn XP
Description and Tags
Molecular Geometry
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
12 Terms
1
New cards
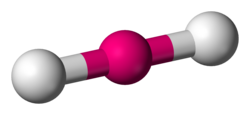
Linear
Lone Pairs: 0
Atoms Touching: 2
Angle: 180
2
New cards
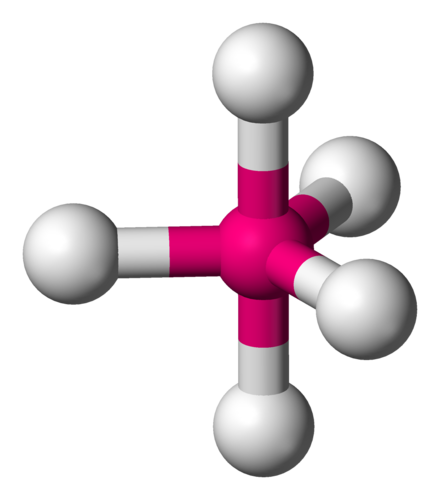
Trigonal Bipyramidal
Lone Pairs: 0
Atoms Touching: 5
Angle: 90 & 120
3
New cards
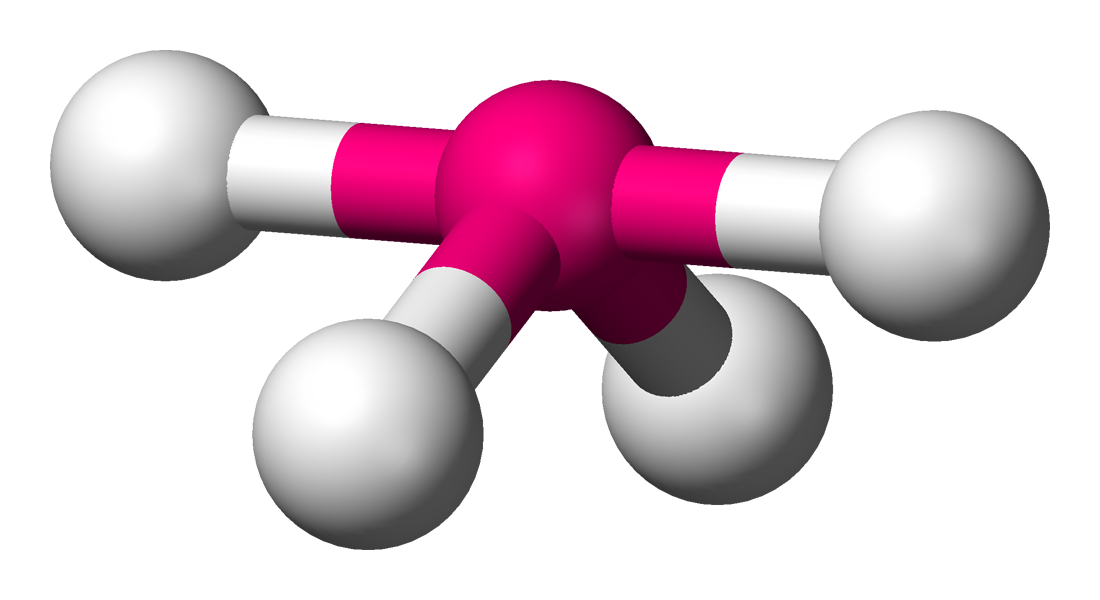
See-saw
Lone Pairs: 1
Atoms Touching: 4
Angle: 120 & 90
4
New cards
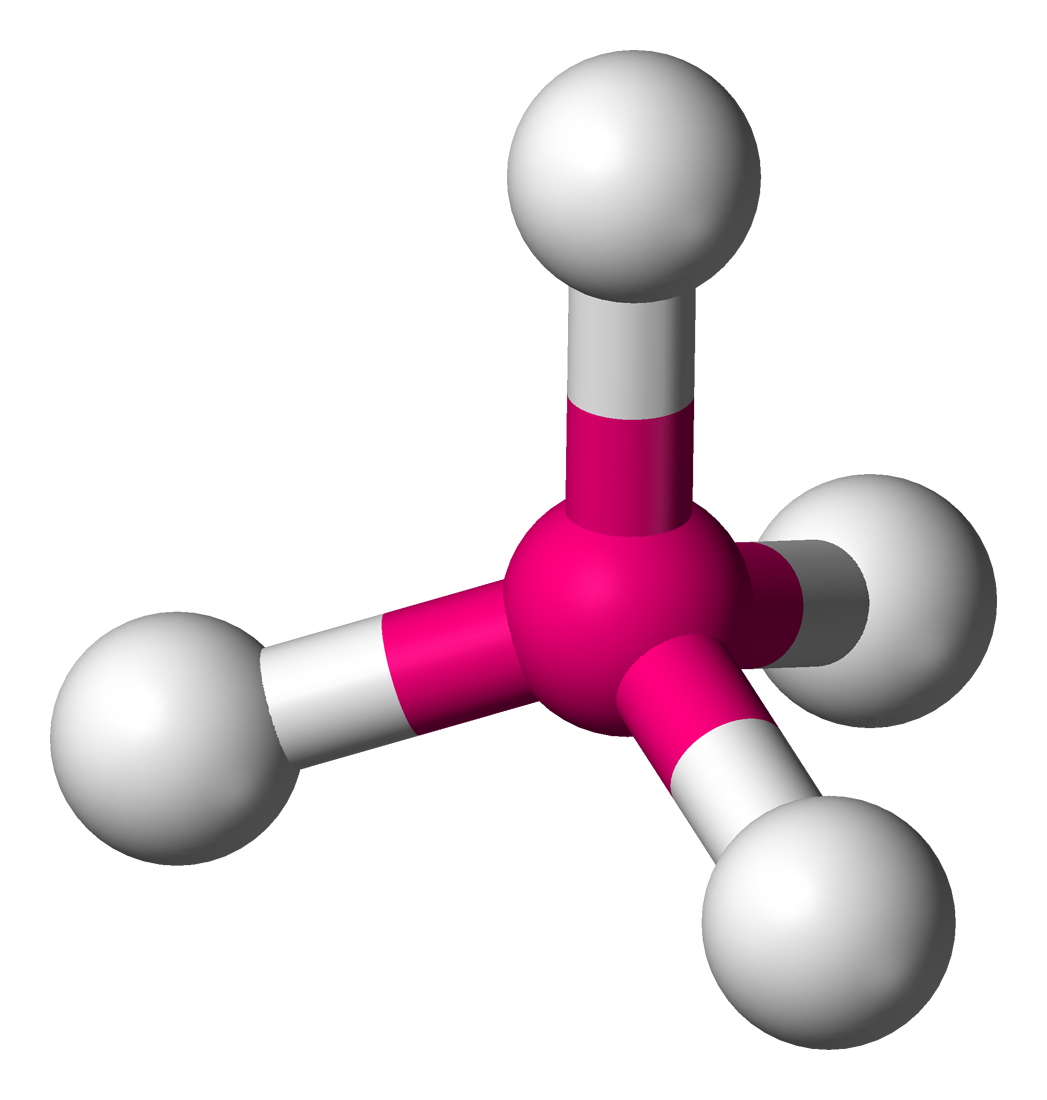
Tetrahydral
Lone Pairs: 0
Atoms Touching: 4
Angle: 109.5
5
New cards
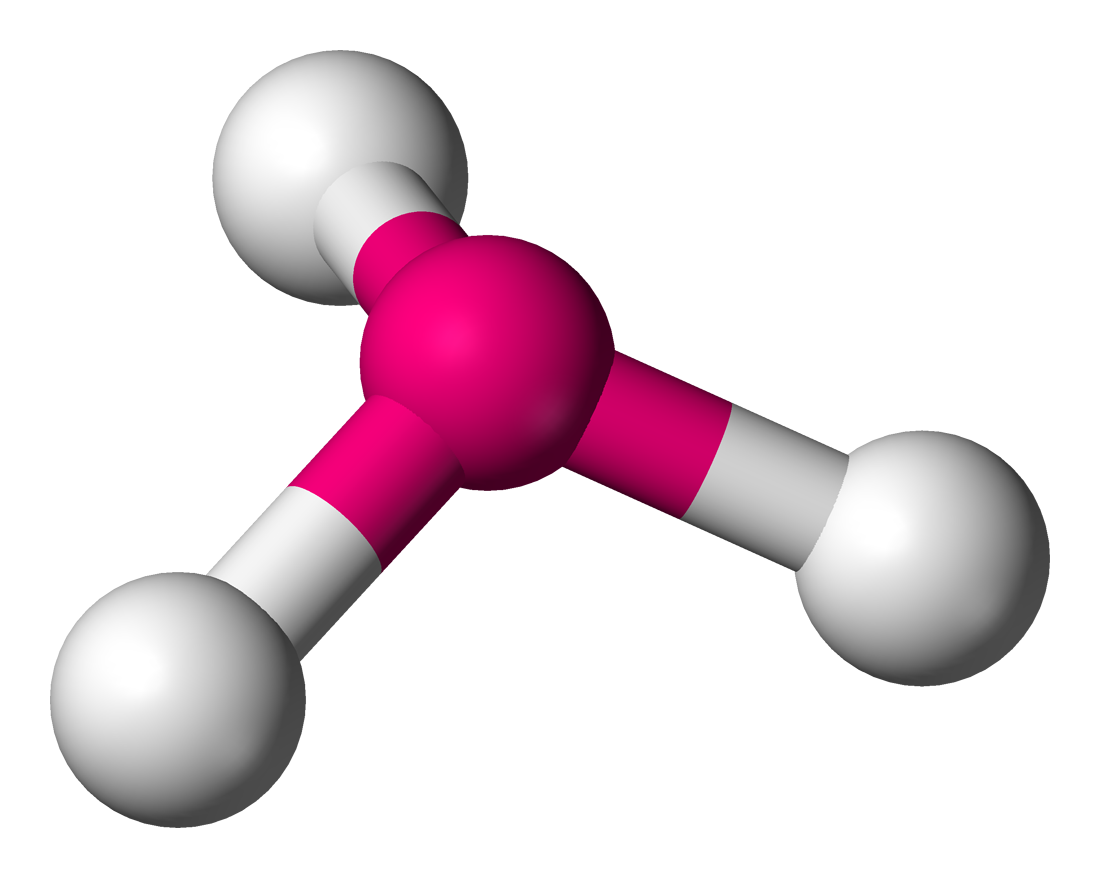
Trigonal Pyramidal
Lone Pairs: 1
Atoms Touching: 3
Angle: 109.5
6
New cards
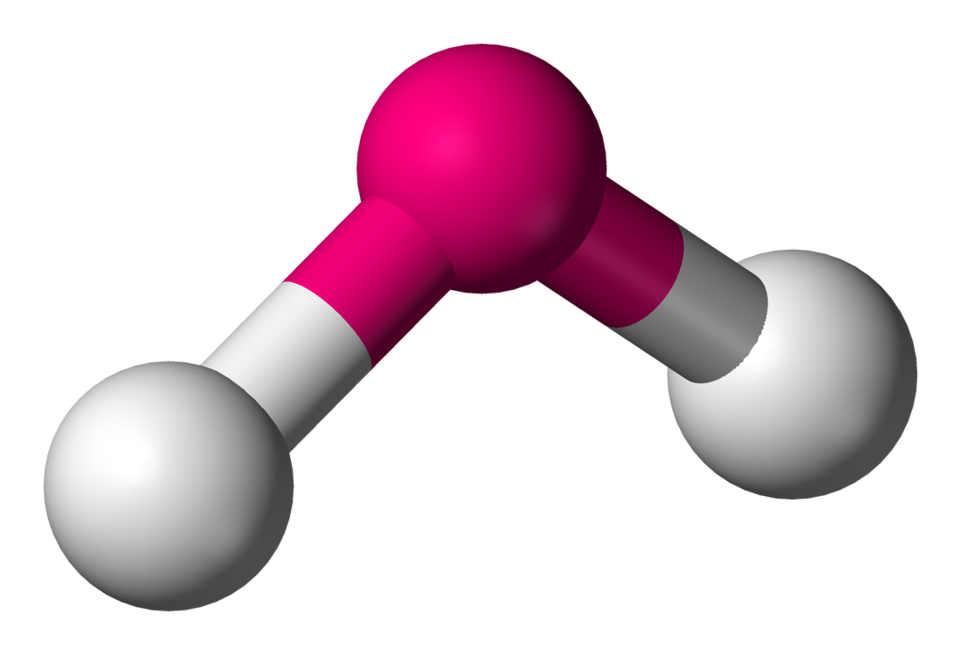
Bent
Lone Pairs: 2
Atoms Touching: 2
Angle:109.5
7
New cards
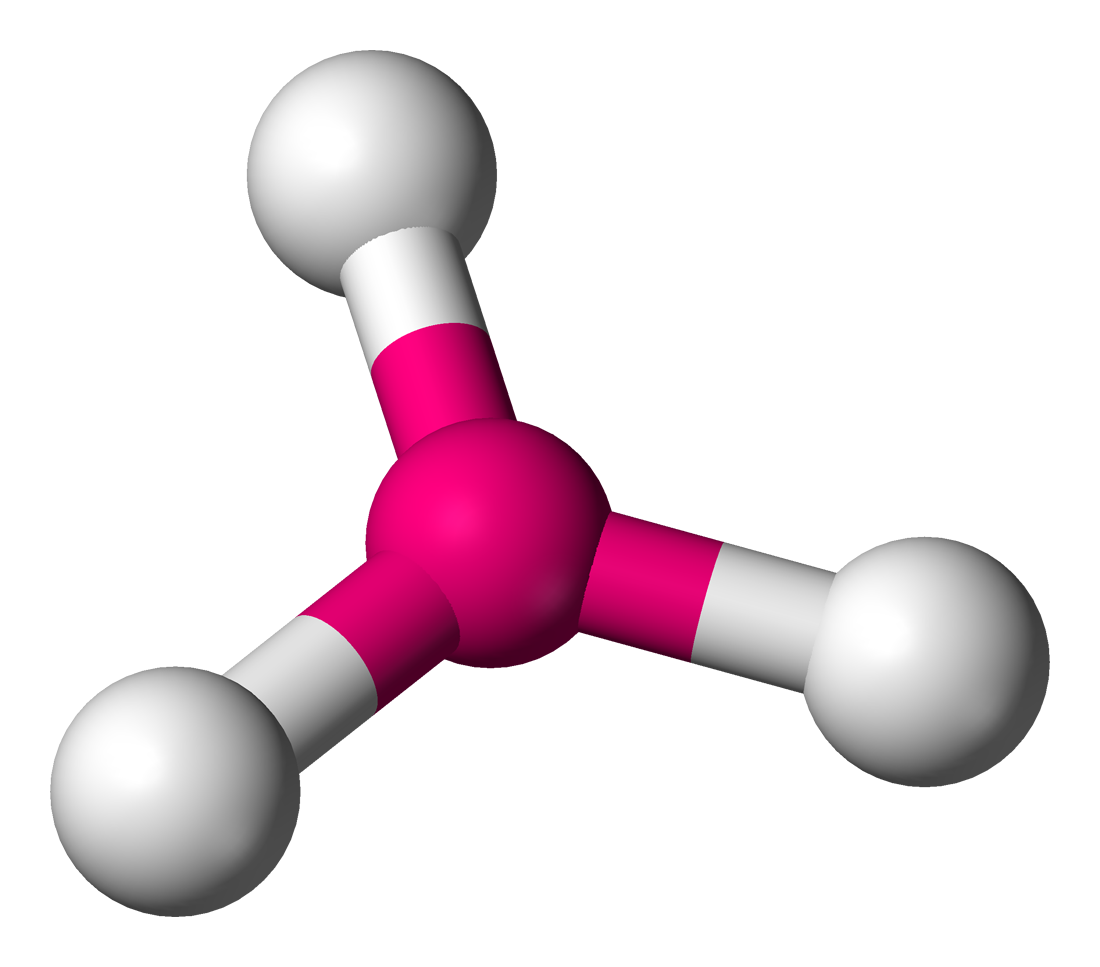
Trigonal Planar
Lone Pairs: 0
Atoms Touching: 3
Angle: 120
8
New cards
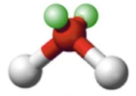
Bent/Angular
Lone Pairs: 1
Atoms Touching: 2
Angle: 120
9
New cards
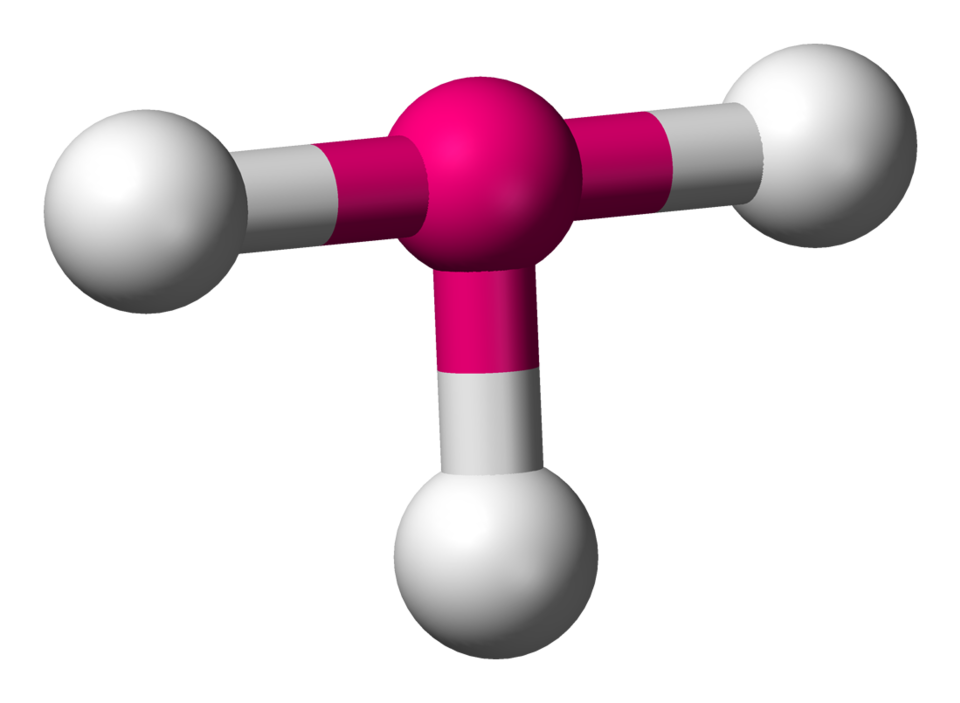
T-Shaped
Lone Pairs: 2
Atoms Touching: 3
Angle: 90
10
New cards
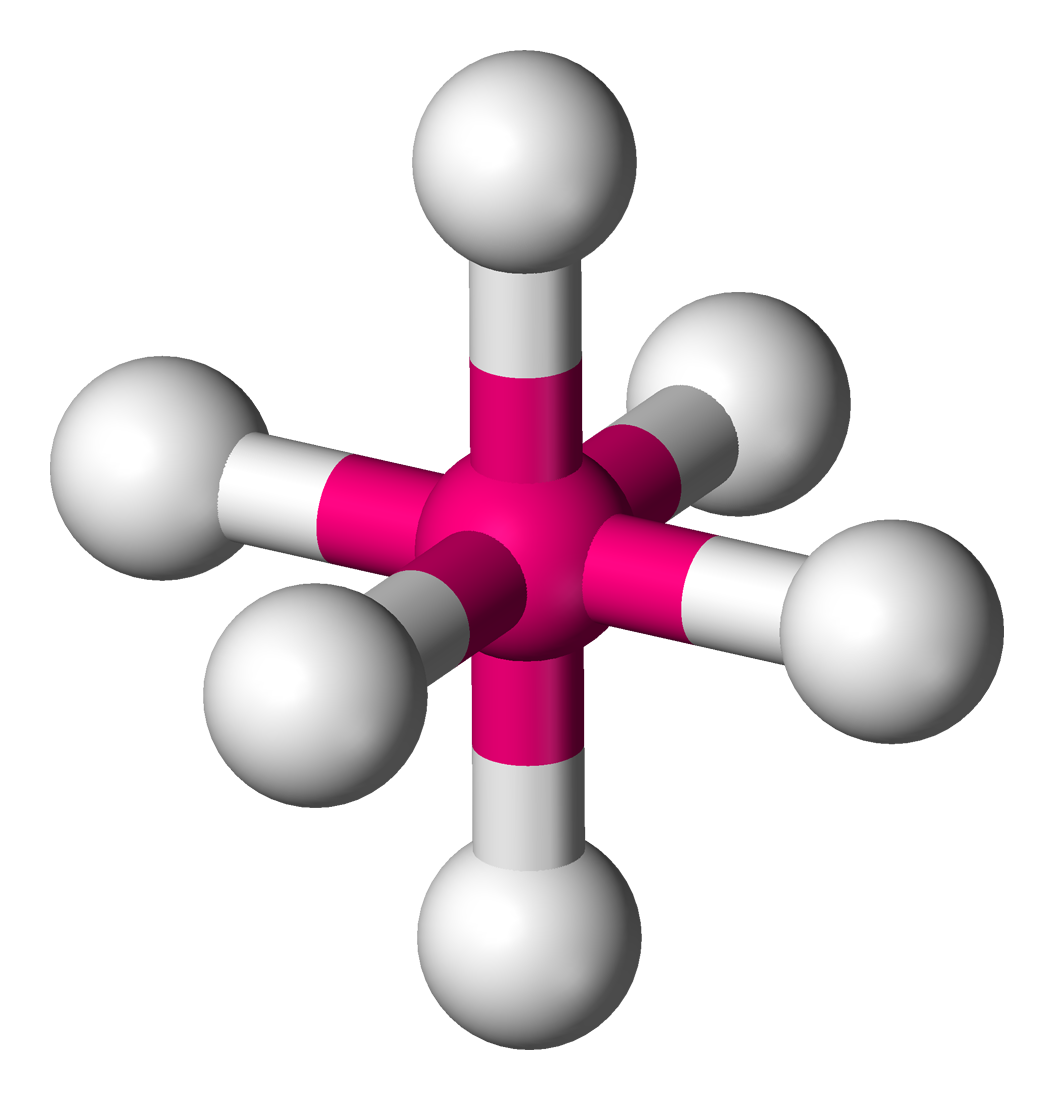
Octahedral
Lone Pairs: 0
Atoms Touching: 6
Angle: 90
11
New cards
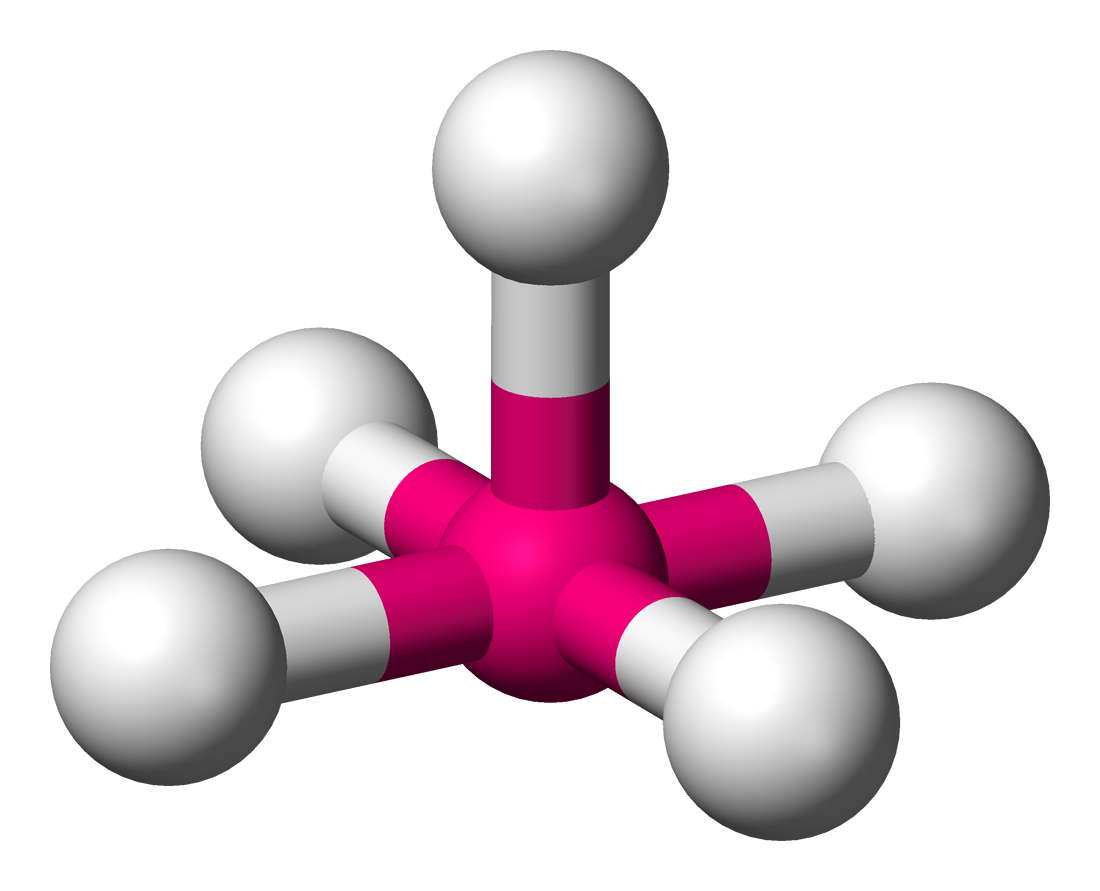
Square Pyramidal
Lone Pairs: 1
Atoms Touching: 5
Angle: 90
12
New cards
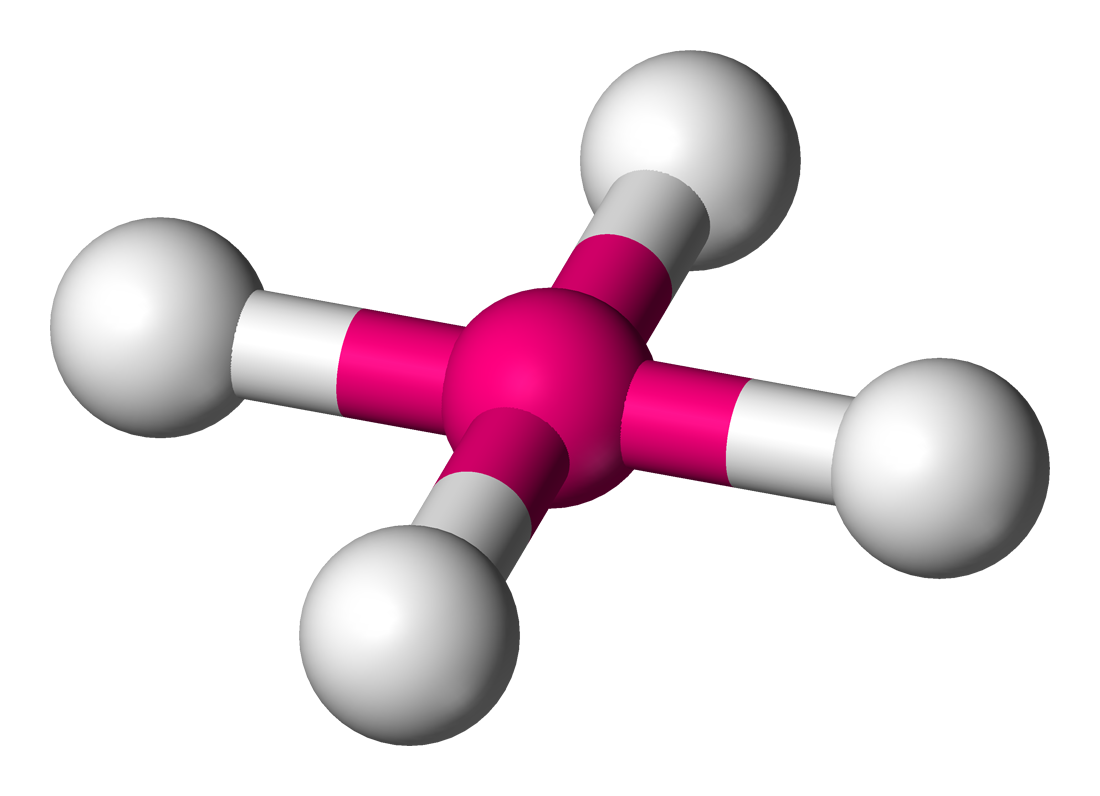
Square Planar
Lone Pairs: 2
Atoms Touching: 4
Angle: 90