Module 2 exam style questions (OCR A A Level Chemistry) Part 2 Electron Structure
1/11
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
12 Terms
Explain why xenon has a lower first ionisation energy than neon.
Xe has a bigger atomic radius OR Xe has more shells
Xe has more shielding
There must be a clear comparison ie more shielding OR increased shielding.
The nuclear attraction decreases
OR Outermost electrons of Xe experience less attraction (to nucleus)
OR Increased shielding / distance outweighs the increased nuclear charge.
Draw a 'dot-and-cross' diagram to show the bonding in NH4+. Show outer electrons only.
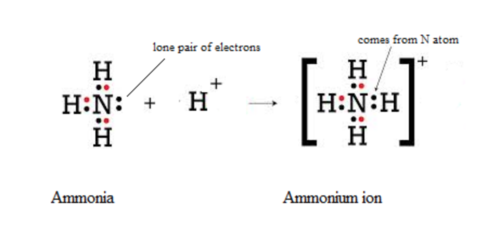
State the shape of, and bond angle in, an NH4+ ion.
tetrahedral 109.5°
Define the term first ionisation energy.
The energy required to remove one electron from each atom in one mole of gaseous atoms
Suggest why the second ionisation energy of oxygen has a greater value than the first ionisation energy of oxygen.
the O+ ion, is smaller than the O atom
OR the electron repulsion/shielding is smaller
OR the proton : electron ratio in the 2+ ion is greater than in the 1+ ion
State what is meant by the term atomic number.
number of protons (in the nucleus)
Explain how the information in the table above provides evidence for two electron shells in ...
Large difference between .. and .. IEs 2 marking a different shell (closer to nucleus)
Write an equation, with state symbols, to represent the second ionisation energy of calcium.
Ca+(g) → Ca2+(g) + e−
Equation with correct charges and 1 electron lost state symbols
Why are the second ionisation energies of calcium and strontium greater than their first ionisation energies?
same number of protons or same nuclear charge attracting less electrons/ electron removed from an ion/ less electron-electron repulsion (not less shielding)/ ion is smaller
Explain why the first and second ionisation energies of strontium are less than those of calcium.
atomic radii of Sr > atomic radii of Ca/
Sr has electrons in shell further from nucleus than Ca/
Sr has electrons in a higher energy level/
Sr has more shells
Therefore less attraction
Sr has more shielding than Ca
The removal of one electron from each atom in 1 mole of gaseous radium atoms is called the .................
First ionisation (energy)
Atoms of radium have a greater nuclear charge than atoms of calcium.
Explain why, despite this, less energy is needed to remove an electron from a radium atom than from a calcium atom.
atomic radii of Ra > atomic radii of Ca/
Ra has electrons in shell further from nucleus than Ca/
Ra has more shells
Ra has more shielding than Ca: 'more' is essential
Ra electron held less tightly/less attraction on electron