Nitrogen Turnover
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
10 Terms
Why do we need nitrogen in our bodies?
Nitrogen ranks the fourth most abundant element of the mass of living cells
amino acids (proteins) have nitrogen
Nucleic acids (DNA/RNA) have nitrogen
Why do we need nitrogen biomolecules?
nitrogen brings charge and polarity and allows for complex intra and intermolecular interactions
peptide bonds in proteins
hydrogen bonds in nucleic acids in proteins
The origin of N and N cycle in biosphere
N is abundant but cannot be utilized directly: N2 is very inert
needs to be converted into ammonia (NH4): nitrogen fixation
done by bacteria
ammonia is further converted to more complex molecules using amino acids as intermediates of N metabolism
Barriers for nitrogen fixation
N2 bond has extremely high energy
The Haber-Bosch Process: N2 + 3H2 → 2NH3; 200 ATM pressure + 400C
Nitrogenase complex: lots of ATP required to make 2NH4; normal pressure + RT
feeds the entire planet
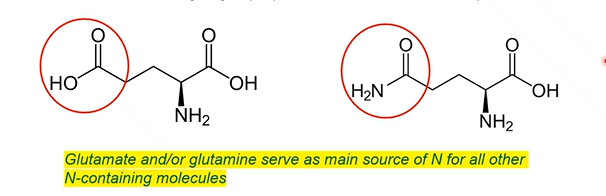
Two critical amino acids to know
Glutamate: 5 carbon amino acid, 2 carboxy groups (acidic)
Glutamine: 5 carbon amino acid, 2 nitrogen groups (amino and amido, net neutral)
main sources of nitrogen
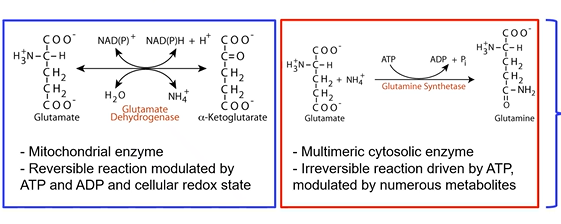
glutamate biochemical reactions
Nitrogen balance (LO1)
The difference between dietary nitrogen intake and excretion
it is very difficult to increase/retain nitrogen-containing compounds in the body just by changing nitrogen input
nitrogen intake is matched by protein turnover and nitrogen excretion
N balance is biochemically orchestrated
the bulk of nitrogen is in proteins
body proteins are predominantly in skeletal muscles
can be supplemented from dietary proteins
body proteins can serve as a major source of energy
Drivers of positive and negative nitrogen balance
Anabolic (regenerative)
testosterone → produced from cholesterol
growth hormone
insulin
Catabolic (degenerative)
cortisol
adrenaline
glucagon