AP Chemistry Exam
5.0(2)
5.0(2)
Card Sorting
1/136
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
137 Terms
1
New cards
atomic number
The same as the number of protons in the nucleus of an element; it is also the same as the number of electrons surrounding the nucleus of an element when it is neutrally charged.
2
New cards
mass number
The sum of an atom's neutrons and protons
3
New cards
isotopes
Atoms of an element with different numbers of neutrons
4
New cards
Avogadro's number
6.022×10²³ particles per one mole
5
New cards
Moles
grams/molar mass
6
New cards
Standard Temperature and Pressure (STP)
Pressure \= 1 atm
Temperature \= 273 K
Temperature \= 273 K
7
New cards
Converting from moles to liters
I mole of gas \= 22.4 L
8
New cards
Moles and Solutions
Moles \= (molarity)(liters of solution)
9
New cards
percent composition (mass percents)
The percent by mass of each element that makes up a compound. It is calculated by dividing the mass of each element or component in a compound by the total molar mass for the substance.
10
New cards
empirical formula
- Represents the simplest ratio of one element to another in a compound
- Start by assuming a 100 g sample
- Convert percentages to grams
- Convert grams into moles
- Divide each mole value by the lowest of the values
- These values become the subscripts
- Start by assuming a 100 g sample
- Convert percentages to grams
- Convert grams into moles
- Divide each mole value by the lowest of the values
- These values become the subscripts
11
New cards
molecular formula
- Determine the molar mass of the empirical formula
- Divide that mass into the molar mass
x \= m/e
x \= molar mass/ empirical mass
- Multiply all subscripts in the empirical formula by the value of x
- Divide that mass into the molar mass
x \= m/e
x \= molar mass/ empirical mass
- Multiply all subscripts in the empirical formula by the value of x
12
New cards
Aufbau principle
States that when building up the electron configuration of an atom, electrons are placed in orbitals, subshells, and shells in order of increasing energy.
13
New cards
Pauli Exclusion Principle
States that the two electrons which share an orbital cannot have the same spin. One electron must spin clockwise, and the other must spin counterclockwise.
14
New cards
Hund's Rule
States that when an electron is added to a subshell, it will always occupy an empty orbital if no one is available. Electrons always occupy orbitals singly if possible and pair up only if no empty orbitals are available.
15
New cards
Coulomb's Law
The amount of energy that an electron has depends on its distance from nucleus of an atom. While on the exam, you will not be required to mathematically calculate the amount of energy a given electron has, you should be able to qualitatively apply Coulomb's Law.
Essentially, the greater the charge of the nucleus, the more energy an electron will have.
Essentially, the greater the charge of the nucleus, the more energy an electron will have.
16
New cards
Quantum Theory
Max Planck figured out that electromagnetic energy is quantized. That is, for a given frequency of radiation (or light), all possible energies are multiples of a certain unit of energy, called a quantum (mathematically, that's E \= hv). So, energy changes do not occur smoothly but rather in small but specific steps.
17
New cards
Energy and Electromagnetic Radiation
ΔE \= hv \= hc/λ
ΔE \= energy change
h \= Planck's constant, 6.626×10⁻³⁴ J∙s
v \= frequency of the radiation
λ \= wavelength of the radiation
c \= the speed of light, 3.00×10⁸ m/s
ΔE \= energy change
h \= Planck's constant, 6.626×10⁻³⁴ J∙s
v \= frequency of the radiation
λ \= wavelength of the radiation
c \= the speed of light, 3.00×10⁸ m/s
18
New cards
Frequency and Wavelength
c \= λv
*Inversely proportional*
c \= speed of light in a vacuum (2.998×10⁸ m/s)
λ \= wavelength of the radiation
v \= frequency of the radiation
*Inversely proportional*
c \= speed of light in a vacuum (2.998×10⁸ m/s)
λ \= wavelength of the radiation
v \= frequency of the radiation
19
New cards
ionization energy
The amount of energy necessary to remove an electron from an atom.
20
New cards
photoelectron spectra (PES)
A chart of the amount of ionization energy for all electrons ejected from a nucleus.
The y-axis describes the relative number of electrons that are ejected from a given energy level.
The x-axis shows the binding energy of those electrons.
The y-axis describes the relative number of electrons that are ejected from a given energy level.
The x-axis shows the binding energy of those electrons.
21
New cards
Gases will most likely act as ideal under what conditions?
High temperature and low pressure
22
New cards
Energy Levels
s-subshell holds two electrons
p-subshell holds six electrons
d-subshell holds 10 electrons
f-subshell holds 14 electrons
p-subshell holds six electrons
d-subshell holds 10 electrons
f-subshell holds 14 electrons
23
New cards
electron configuration
The complete description of the energy level and subshell that each electron on an element inhabits
24
New cards
Heisenberg Uncertainty Principle
States that it is impossible to know both the momentum of an electron at a particular instant.
25
New cards
Atomic Radius Trends
Atomic radius decreases across a period
Atomic radius increases down a group
Cations are smaller than their atoms
Ions are larger than their atoms
Atomic radius increases down a group
Cations are smaller than their atoms
Ions are larger than their atoms
26
New cards
Ionization energy
The energy required to remove an electron from an atom.
27
New cards
Electronegativity
Refers to how strongly the nucleus of an atom attracts the electrons of other atoms in a bond.
28
New cards
Periodic Trends
Across the periods
- atomic radius decreases
- ionization energy increases
- electronegativity increases
Down the periods
- atomic radius increases
- ionization energy decreases
- electronegativity decreases
- atomic radius decreases
- ionization energy increases
- electronegativity increases
Down the periods
- atomic radius increases
- ionization energy decreases
- electronegativity decreases
29
New cards
Ionic Bonds
An ionic solid is held together by the electrostatic attractions between ions that are next to one another in a lattice structure.
Occurs between a metal and a nonmetal; electrons are not shared, they are given up by one atom and accepted by another.
Substances with ionic bonds are usually solids at room temperature and have very high melting and boiling points.
Ex. NaCl
Occurs between a metal and a nonmetal; electrons are not shared, they are given up by one atom and accepted by another.
Substances with ionic bonds are usually solids at room temperature and have very high melting and boiling points.
Ex. NaCl
30
New cards
Factors Affecting Melting Points of Ionic Substances
1. Charge on ions - a greater charge leads to a greater bond energy
Ex. MgO will have a higher melting point than NaCl
2. Size of ions - smaller ions will have greater attraction
Ex. LiF will have a greater melting KBr
Ex. MgO will have a higher melting point than NaCl
2. Size of ions - smaller ions will have greater attraction
Ex. LiF will have a greater melting KBr
31
New cards
Interstitial alloys
Metal atoms with two different radii combine
Ex. In steel, much smaller carbon atoms occupy the interstices of the iron atoms
Ex. In steel, much smaller carbon atoms occupy the interstices of the iron atoms
32
New cards
Substitutional alloy
Forms between atoms of similar radii
Ex. Atoms of zinc are substituted with copper atoms to create an alloy
Ex. Atoms of zinc are substituted with copper atoms to create an alloy
33
New cards
Covalent bonding
Bonding in which two atoms share electrons. Each atom counts the shared electrons as part of its valence shell to achieve complete outer shells.
The first covalent bond formed between two atoms is called a sigma bond.
The first covalent bond formed between two atoms is called a sigma bond.
34
New cards
Single bonds
Bond designation: One sigma
Bond order: One
Bond length: Longest
Bond energy: Least
Bond order: One
Bond length: Longest
Bond energy: Least
35
New cards
Double Bond
Bond designation: One sigma and one pi
Bond order: Two
Bond length: Intermediate
Bond energy: Intermediate
Bond order: Two
Bond length: Intermediate
Bond energy: Intermediate
36
New cards
Triple Bond
Bond designation: One sigma and two pi
Bond order: Three
Bond length: Shortest
Bond energy: Greatest
Bond order: Three
Bond length: Shortest
Bond energy: Greatest
37
New cards
Network (Covalent) Bonds
In a network solid, atoms are held together in a lattice of covalent bonds.
They are very hard and have very high melting and boiling points.
Ex. The most commonly seen network solids are compounds of carbon (such as diamond or graphite) and silicon (SiO₂ quartz)
They are very hard and have very high melting and boiling points.
Ex. The most commonly seen network solids are compounds of carbon (such as diamond or graphite) and silicon (SiO₂ quartz)
38
New cards
Hydrogen Bonds
Much stronger than dipole-dipole forces.
Substances that have hydrogen bonds have higher melting and boiling points. Ex. water, H₂O and ammonia, NH₃
*Water is less dense as a solid than as a liquid because its hydrogen bonds force the molecules in ice to form a crystal structure, which keeps them apart than they are in the liquid form
Substances that have hydrogen bonds have higher melting and boiling points. Ex. water, H₂O and ammonia, NH₃
*Water is less dense as a solid than as a liquid because its hydrogen bonds force the molecules in ice to form a crystal structure, which keeps them apart than they are in the liquid form
39
New cards
Dipole-Dipole Forces
Forces that occur when the positive end of one polar molecule is attracted to the negative end of another polar molecule.
Molecules with greater polarity have higher melting and boiling points.
Dipole-dipole attractions, however, are relatively weak, and these substances melt and boil at very low temperatures.
Molecules with greater polarity have higher melting and boiling points.
Dipole-dipole attractions, however, are relatively weak, and these substances melt and boil at very low temperatures.
40
New cards
London Dispersion Forces
Forces that occur between all molecules.
These very weak attractions occur because of the random motions of electrons on atoms within molecules. At a given moment, a nonpolar molecule might have more electrons on one side than on the other, given it an instantaneous polarity.
Molecules with more electrons will experience will experience greater London dispersion forces, and therefore have generally higher melting and boiling points.
London forces are even weaker than dipole-dipole forces, so substances that have only London dispersion forces melt and boil at extremely low temperatures and tend to be gases at room temperature.
These very weak attractions occur because of the random motions of electrons on atoms within molecules. At a given moment, a nonpolar molecule might have more electrons on one side than on the other, given it an instantaneous polarity.
Molecules with more electrons will experience will experience greater London dispersion forces, and therefore have generally higher melting and boiling points.
London forces are even weaker than dipole-dipole forces, so substances that have only London dispersion forces melt and boil at extremely low temperatures and tend to be gases at room temperature.
41
New cards
Different types of bonds and their relative melting and boiling points (from highest to lowest)
1. Network Covalent Bonds
2. Ionic Bonds (based on Coulombic attraction)
a. Greater Ion Charge
b. Smaller Atom Size
3. Covalent Bonds (based on molecular polarity)
a. Hydrogen Bonds
b. Non-Hydrogen Bond Dipoles
c. London Dispersion Forces (temporary dipoles)
i. Large molecules are more polarizable because they have more electrons.
2. Ionic Bonds (based on Coulombic attraction)
a. Greater Ion Charge
b. Smaller Atom Size
3. Covalent Bonds (based on molecular polarity)
a. Hydrogen Bonds
b. Non-Hydrogen Bond Dipoles
c. London Dispersion Forces (temporary dipoles)
i. Large molecules are more polarizable because they have more electrons.
42
New cards
How intermolecular forces affect the phase of a substance
1. Substances with weak intermolecular forces (LD), tend to be gases at room temperature.
2. Substances with strong intermolecular forces (HB) tend to be liquids at room temperature.
3. Because ionic bonds are generally significantly stronger tan intermolecular forces in covalent molecules, ionic substances are usually solid at room temperatures.
*Ionic substances do not experience intermolecular forces.*
2. Substances with strong intermolecular forces (HB) tend to be liquids at room temperature.
3. Because ionic bonds are generally significantly stronger tan intermolecular forces in covalent molecules, ionic substances are usually solid at room temperatures.
*Ionic substances do not experience intermolecular forces.*
43
New cards
Vapor Pressure
Arises from the fact that molecules inside a liquid are in constant motion. If those molecules hit the surface of the liquid with enough kinetic energy, they can escape the intermolecular forces holding them to the other molecules and transition them into the gas phase.
This is not to be confused with a liquid boiling. In order for vaporization to occur, no outside energy needs to be added.
This is not to be confused with a liquid boiling. In order for vaporization to occur, no outside energy needs to be added.
44
New cards
Resonance Forms
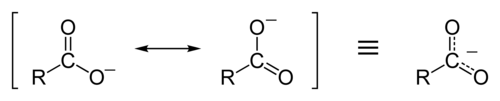
45
New cards
Incomplete Octets
Some atoms are stable with less than eight electrons in their outer shell.
\
\- Hydrogen only requires two electrons
\
\- Boron is considered to be stable with only six electrons, as in BF₃
\
\- Hydrogen only requires two electrons
\
\- Boron is considered to be stable with only six electrons, as in BF₃
46
New cards
Expanded Octets
In molecules that have d subshells available, the central atom can have more than eight valence electrons, but never more than twelve.
- PCl₅
- SF₄
- XeF₄
- PCl₅
- SF₄
- XeF₄
47
New cards
Formal Charge
If more than one valid Lewis structure exists for a molecule, formal charge can be used to determine the more likely structure.
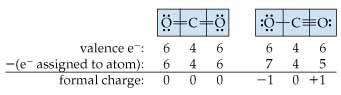
48
New cards
Standard Enthalpy of Formation
The amount of heat lost or gained when one mole of a compound is formed from its constituent elements.
49
New cards
Enthalpy Change (Hess's Law for ΔH)
The enthalpy change for a reaction is equal to the sum of the enthalpy of formation of all the products minus the sum of the enthalpy of formation of all the reactants.
50
New cards
Valence Shell Electron Pair Repulsion Model
Electrons repel each other, so when atoms come together to form a molecule, the molecule will assume the shape that keeps its different electron pairs as far apart as possible.
51
New cards
Linear Geometry
- Central atom with 2 electron pairs
- Zero lone pairs
- sp hybridization
- Ex. BeCl₂ and CO₂
B - A - B
- Zero lone pairs
- sp hybridization
- Ex. BeCl₂ and CO₂
B - A - B
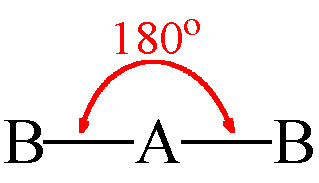
52
New cards
Trigonal Planar Geometry
- Central atom with three electron pairs
- Zero lone pairs
- sp² hybridization
- 120° bond angles
- Ex. BF₃, SO₃, NO₃⁻, CO₃²⁻
- Zero lone pairs
- sp² hybridization
- 120° bond angles
- Ex. BF₃, SO₃, NO₃⁻, CO₃²⁻
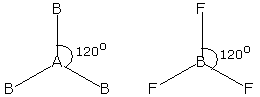
53
New cards
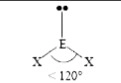
Bent Geometry
- Central atom with three electron pairs
- One lone pair
- sp² hybridization
- 120° bond angle
- Ex. SO₂
- One lone pair
- sp² hybridization
- 120° bond angle
- Ex. SO₂
54
New cards
Specific Heat Capacity
The amount of heat required to raise the temperature of one mass unit of a substance by 1.00°C
55
New cards
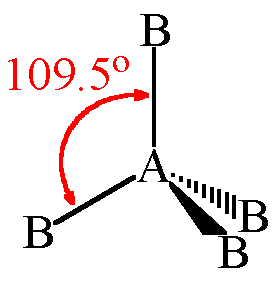
Tetrahedral Geometry
- Central atom has four electron pairs
- Zero lone pairs
- sp³ hybridization
- 109.5° bond angle
- Ex. CH₄, NH₄⁺, ClO₄⁻, SO₄²⁻, PO₄³⁻
- Zero lone pairs
- sp³ hybridization
- 109.5° bond angle
- Ex. CH₄, NH₄⁺, ClO₄⁻, SO₄²⁻, PO₄³⁻
56
New cards
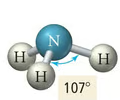
Trigonal Pyramidal Geometry
- Central atom has four electron pairs
- One lone pair
- sp³ hybridization
- 109.5° bond angle
- Ex. NH₃, PCl₃, AsH₃, SO₃²⁻
- One lone pair
- sp³ hybridization
- 109.5° bond angle
- Ex. NH₃, PCl₃, AsH₃, SO₃²⁻
57
New cards
Bent Geometry
- Central atom has four electron pairs
- two lone pair
- sp³ hybridization
- 109.5° bond angle
- Ex. H₂O, OF₂, NH₂⁻
- two lone pair
- sp³ hybridization
- 109.5° bond angle
- Ex. H₂O, OF₂, NH₂⁻

58
New cards
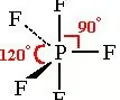
Trigonal Bipyramidal Geometry
- Central atom has 5 electron pairs
- Zero lone pairs
- dsp³ hybridization
- Ex. PCl₅, PF₅
- Zero lone pairs
- dsp³ hybridization
- Ex. PCl₅, PF₅
59
New cards
Folded square, seesaw, distorted tetrahedron geometry
- Central atom has 5 electron pairs
- One lone pair
- dsp³ hybridization
- Ex. SF₄, IF₄⁺
- One lone pair
- dsp³ hybridization
- Ex. SF₄, IF₄⁺
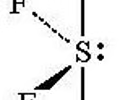
60
New cards
T-Shaped Geometry
- Central atom has 5 electron pairs
- Two lone pairs
- dsp³ hybridization
- Ex. ClF₃, ICl₃
- Two lone pairs
- dsp³ hybridization
- Ex. ClF₃, ICl₃
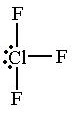
61
New cards
Linear Geometry
- Central atom has 5 electron pairs
- Three lone pairs
- dsp³ hybridization
- Ex. XeF₂, I₃⁻
- Three lone pairs
- dsp³ hybridization
- Ex. XeF₂, I₃⁻

62
New cards
Octahedral Geometry
- Central atom has 6 electron pairs
- Zero lone pairs
- d²sp³ hybridization
- Ex. SF₆
- Zero lone pairs
- d²sp³ hybridization
- Ex. SF₆
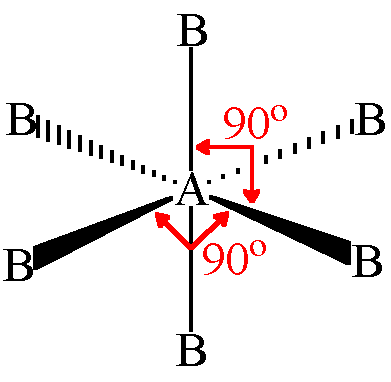
63
New cards
Square Pyramidal
- Central atom has 6 electron pairs
- One lone pair
- d²sp³ hybridization
- Ex. BrF₅, IF₅
- One lone pair
- d²sp³ hybridization
- Ex. BrF₅, IF₅
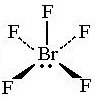
64
New cards
Square Planar
- Central atom has 6 electron pairs
- Two lone pairs
- d²sp³ hybridization
- Ex. XeF₄, ICl₄⁻
- Two lone pairs
- d²sp³ hybridization
- Ex. XeF₄, ICl₄⁻
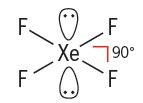
65
New cards
Entropy
A measure of molecular randomness, or disorder
66
New cards
Kinetic Molecular Theory
- The kinetic energy of an ideal gas is directly proportional to its absolute temperature: The greater the temperature, the greater the average kinetic energy of the gas molecules.
- There are no forces of attraction between the gas molecules in an ideal gas.
- Gas molecules are in constant motion, colliding with one another and with the walls of their container without losing any energy.
- There are no forces of attraction between the gas molecules in an ideal gas.
- Gas molecules are in constant motion, colliding with one another and with the walls of their container without losing any energy.
67
New cards
The Ideal Gas Equation
PV \= nRT
R \= .08206 Latm/molK
R \= .08206 Latm/molK
68
New cards
If volume is constant
As pressure increases, temperature increases
69
New cards
Boyle's Law (If temperature is constant)
As pressure increases, volume decreases, and vice versa
70
New cards
Charles' Law (If pressure is constant)
As temperature increases, volume increases
71
New cards
Dalton's Law
Ptotal \= Pa + Pb + Pc + ...
72
New cards
Partial Pressure
Pa \= (Ptotal)(Xa)
Xa \= moles of gas A/total moles of gas
Xa \= moles of gas A/total moles of gas
73
New cards
Deviations From Ideal Behavior
At low temperature and/or high pressure, gases behave in a less-than-ideal manner. This is because the assumptions made in the kinetic molecular theory become invalid.
74
New cards
Density
D \= m/v
75
New cards
Molarity (M)
Expresses the concentration of a solution in terms of volume.
M \= moles of solute / liters of solution
M \= moles of solute / liters of solution
76
New cards
Mole Fraction
Mole fraction gives the fraction of moles of a given substance (S) out of the total moles present in a sample.
Mole Fraction (Xa) \= moles of substance S / total number of moles in solution
Mole Fraction (Xa) \= moles of substance S / total number of moles in solution
77
New cards
Solutes and Solvents
"Like dissolves like"
A basic rule to remember which solutes will dissolve in which solvents.
Polar or ionic solutes (such as salt) will dissolve in polar solvents (such as water).
Nonpolar solutes (such as oils) are best dissolved in nonpolar solvents.
When an ionic substance dissolves, it breaks up into ions in a process called dissociation. Free ions in a solution are called electrolytes because they can conduct electricity.
A basic rule to remember which solutes will dissolve in which solvents.
Polar or ionic solutes (such as salt) will dissolve in polar solvents (such as water).
Nonpolar solutes (such as oils) are best dissolved in nonpolar solvents.
When an ionic substance dissolves, it breaks up into ions in a process called dissociation. Free ions in a solution are called electrolytes because they can conduct electricity.
78
New cards
Oxidation
Electron loss
79
New cards
Reduction
Electron gain
80
New cards
Oxidizing agent
Reactant that is reduced (gains electrons)
81
New cards
Reducing agent
Reactant that is oxidized (loses electrons)
82
New cards
Spontaneous
Not requiring an outside source of energy to proceed
83
New cards
Graham's Law
The rate of diffusion of a gas molecule is inversely proportional to the square root of that molecule's mass.
84
New cards
Gibbs Free Energy
The amount of energy in a system that is available to do useful work.
-ΔG is spontaneous
+ΔG is nonspontaneous
When ΔG \= 0 the reaction is at equilibrium
-ΔG is spontaneous
+ΔG is nonspontaneous
When ΔG \= 0 the reaction is at equilibrium
85
New cards
Buffer Solution
A solution consisting of a weak acid plus its conjugate base or a weak base plus its conjugate acid.
This solution resists changes to its pH
This solution resists changes to its pH
86
New cards
Crystalline Solids
Solid that has its atoms arranged in an orderly way.
87
New cards
Amorphous Solid
Solids whose particles have no orderly pattern.
88
New cards
Solubility Rules
1. Compounds with an alkali metal cation (Na⁺, Li⁺, K⁺, etc) or an ammonium cation (NH₄⁺) are always soluble.
2. Compounds with a nitrate (NO₃⁻) anion are always soluble.
2. Compounds with a nitrate (NO₃⁻) anion are always soluble.
89
New cards
Enthalpy Change in Bonds
When bonds are broken, energy is released.
When bonds are formed, energy is absorbed.
When bonds are formed, energy is absorbed.
90
New cards
Exothermic Reactions
If the products have stronger bonds than reactants, then the products have lower enthalpy than the reactants and are more stable; in this case, energy is released by the reaction, or the reaction is exothermic.
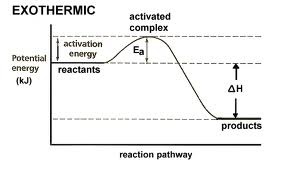
91
New cards
Endothermic Reactions
If the products have weaker bonds than the reactants, then the products have higher enthalpy than the reactants and are less stable; in this case, energy is absorbed by the reaction, or the reaction is exothermic.
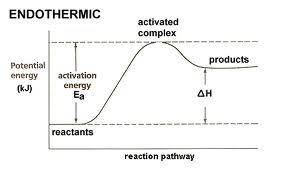
92
New cards
Activation Energy
The amount of energy required to reach the transition state, the highest point on the graph. At this point, all reactant bonds have been broken, but no product bonds have been formed, so this is the point in the reaction with the highest energy and lowest stability.
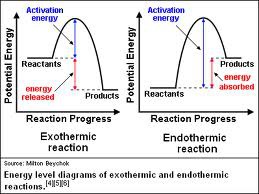
93
New cards
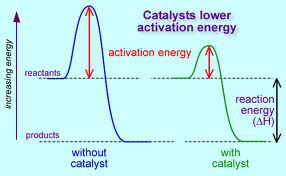
Catalysts
Speed up a reaction by providing the reactants with an alternate pathway that has a lower activation energy.
A catalyst lowers the activation energy, but it has no effect on the energy of the reactants, the energy of the products, or the ΔH of the reaction.
A catalyst lowers the activation energy, but it has no effect on the energy of the reactants, the energy of the products, or the ΔH of the reaction.
94
New cards
Galvanic Cell (Voltaic Cell)
In a galvanic cell, a favored redox reaction is used to generate a flow of current.
Two half-reactions take place in separate chambers, and the electrons that are released by the oxidation reaction pass through a wire to the chamber where they are consumed in the reduction reaction. That's how the current is created.
If the concentration of the products in a voltaic cell increases, the voltage decreases. If the concentration of the reactants increases, the voltage increases.
Two half-reactions take place in separate chambers, and the electrons that are released by the oxidation reaction pass through a wire to the chamber where they are consumed in the reduction reaction. That's how the current is created.
If the concentration of the products in a voltaic cell increases, the voltage decreases. If the concentration of the reactants increases, the voltage increases.
95
New cards
Redox in a Galvanic Cell
Oxidation takes place at the anode
Reduction takes place at the cathode
Reduction takes place at the cathode
96
New cards
Cathode
Where reduction occurs and the solution is becoming less positively charged, the positive cations from the salt bridge solution flow into the half-cell.
97
New cards
Anode
Where oxidation occurs and the solution is becoming more positively charged, the negative anions from the salt bridge solution flow into the half-cell.
98
New cards
Solving Electroplating Problems
1. If you know the current and time, you can calculate the charge in coulombs.
I \= q/t
I \= current (amperes, A)
q \= charge (coulombs, C)
t \= time (seconds, s)
2. Once you know the charge in coulombs, you know how many electrons were involved in the reaction.
moles of electrons \= (coulombs) / (96,500 coulombs/mol)
3. When you know the number of moles of electrons and you know the half-reaction for the metal, you can find out how many moles of metal plated out.
4. Once you know the number of moles of the metal, you can convert this to the number of grams of the metal.
I \= q/t
I \= current (amperes, A)
q \= charge (coulombs, C)
t \= time (seconds, s)
2. Once you know the charge in coulombs, you know how many electrons were involved in the reaction.
moles of electrons \= (coulombs) / (96,500 coulombs/mol)
3. When you know the number of moles of electrons and you know the half-reaction for the metal, you can find out how many moles of metal plated out.
4. Once you know the number of moles of the metal, you can convert this to the number of grams of the metal.
99
New cards
First Order Rate Laws
Rate \= k[A]
The rate law for a first order reaction uses natural logarithms.
The use of natural logarithms in the rate law creates a linear graph comparing concentration and time.
The slope of the line is given by -k and the y-intercept is given by ln[A]₀
The rate law for a first order reaction uses natural logarithms.
The use of natural logarithms in the rate law creates a linear graph comparing concentration and time.
The slope of the line is given by -k and the y-intercept is given by ln[A]₀
![Rate \= k[A]
The rate law for a first order reaction uses natural logarithms.
The use of natural logarithms in the rate law creates a linear graph comparing concentration and time.
The slope of the line is given by -k and the y-intercept is given by ln[A]₀](https://knowt-user-attachments.s3.amazonaws.com/b04dca755f234e64b5e0b7c7925536c1.jpeg)
100
New cards
Half-life
Describes the amount of time it takes for half of a sample to react.