BLOOD DONATION LAB
1/171
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
172 Terms
Donor Selection
its purpose is to to assess the suitability of an individual to be a blood
donor so that blood donation is safe for the donor and the blood products derived from this donation are safe for the recipients.
Will a donation of approximately 450 mL of whole blood be harmful to the donor?
Could blood drawn from this donor at this time potentially transmit a disease to the recipient?
questions that are answered when you assess the medical history information and physical examination
Registration
Health History Interview
Physical Exam
three phases of donor screening
Registration
this process includes documenting information that fully identifies the
donor on an individual donation registration record.
True
T or F
Registration should also include prescreening for donor eligibility status.
Donor Deferral Registry (DDR) or Deferred Donor Directory (DDD)
list of donors who have been deferred from donating blood on a previous donation or donation attempt.
at least 16 yo (<18 needs parental consent)
minimum age requirement for donating blood
16-60 yo
range of age acceptance for new donors
16-70 yo
range of age acceptance for regular donors
8 weeks (2 months)
time elapsed between whole blood donations
12 weeks (3 months)
time elapsed between whole blood donations according to DOH-NVBSP
16 weeks (4 months)
time elapsed after two-unit red cell collection
4 weeks (1 month)
time elapsed after infrequent plasmapheresis
>2 days
time elapsed after plasmapheresis, plateletpheresis, or leukapheresis
Allogenic Donation
donation for use by the general patient population.
Directed Donation
donation reserved for use by a specific patient.
Autologous donation
donation by a donor reserved for the donor’s later use.
Apheresis donation
donation of a specific component of the blood
parts of the whole blood that are not retained are returned to the
donor.
AABB Standards
mandates that informed consent of allogeneic, autologous, and apheresis donors be obtained before donation.
Health History
used to protect both the donor during the donation process and the patient receiving the blood.
questions are asked in an environment that provides confidentiality and encourages the donor to answer truthfully.
True
T or F
Self-administered interview formats have been shown to yield more information regarding HIV high-risk behavior.
questions intended to protect the donor
questions intended to protect the recipient
two categories of the questions during health history interview
Cold
Influenza
headache
Nausea
pregnant
conditions for protection of the donor
conditions that could get a donor to be temporarily deferred
exposure to bloodborne diseases
medications, vaccinations, high-risk activities
viral markers
conditions related for questions that protects the recipient
Temporary Deferral
Indefinite Deferral
Permanent Deferral
types of deferral
Temporary Deferral
Prospective donor is unable to donate blood for a limited period of time.
Malaria
History of residence (lived longer than 5 consecutive years) in countries considered malaria-endemic
Acitretin (Soriatane) therapy
3 years temporary deferral
Mucous membrane exposure to blood
Nonsterile skin or needle penetration
Sexual contact with an individual with viral hepatitis/HIV/Hepa B surface antigen
Incarceration in a correctional institution for longer than 72 consecutive
hours
History of syphilis or gonorrhea
Military personnel with history of travel to Iraq in 2003 (Leishmaniasis)- for 1 year from departure
Transfusion of blood, components, human tissue, plasma-derived clotting
factor concentrates
Human diploid cell–rabies vaccine after animal bite
History of travel to a malaria-endemic area
Receipt of Hepatitis B immune globulin
Receipt of tetanus immunoglobulin
Receipt of unlicensed vaccines
12 month (1 year) deferral
West Nile Virus (WNV)
120 days deferral
Dutasteride (Avodart, Jalyn) therapy
6 months deferral
Pregnancy
Gamma Globulin for contact with Hepatitis A
6 weeks deferral
Receipt of German measles (rubella) vaccine
Receipt of Varicella-zoster (chickenpox) vaccine
Isotretinoin (Accutane, Amnesteem, Claravis, Sotret) therapy
4 weeks (1 month) deferral
Measles (rubeola) vaccine
Mumps vaccine
Polio (oral) vaccine
Typhoid (oral) vaccine
Yellow fever vaccine
Clopidogrel (Plavix), Ticlopidine (Ticlid) therapy (for platelet donors)
2 weeks (14 days) deferral
Warfarin (Coumadin) therapy
1 week deferral
Aspirin therapy
Piroxicam (Feldene) therapy
2 days deferral
✓ Viral hepatitis after 11th birthday
✓ Repeat reactive test for anti-HBc on more than one occasion
✓ Clinical or laboratory evidence of HCV, HTLV, or HIV infection
✓ Previous donation associated with hepatitis, HIV, or HTLV transmission
✓ Behavioral risk factors for HIV infection according to current FDA guidance
✓ History of Babesiosis or Chagas’ disease
✓ Stigma of parenteral drug use
✓ Injection of nonprescription drugs
✓ Risk of vCJD according to current FDA guidelines
Receipt of bovine insulin
Family history of CJD
Dura mater transplant
Receipt of human pituitary-derived growth hormone
✓ History of a bleeding disorder / blood disorder such as:
Hemophilia, von Willebrand disease, sickle cell anemia, thalassemia,
Kaposi’s sarcoma, polycythemia, or a history of receiving clotting factor
concentrates
✓ History of cancer, leukemia, or lymphoma
Exceptions include basal or squamous cell cancer, carcinoma in situ of the
cervix, and papillary thyroid carcinoma that has been surgically removed.
indefinite deferral
Indefinite Deferral
Prospective donor is unable to donate blood for someone else for an unspecified period of time due to current regulatory requirements.
Indefinite Deferral
this donor would not be able to donate blood for as long as these requirements are in force or until the current requirement changes
These donors may be eligible to donate autologous blood.
Permanent Deferral
prospective donor will never be eligible to donate blood for someone else
These donors may be eligible to donate autologous blood.
HBsAg, HIV or Hepatitis C
Xenotransplantation
Receipt of a dura mater (or brain covering) graft
Etretinate (Tegison) therapy
permanent deferral
✓ Anthrax
✓ Cholera
✓ DPT
✓ Influenza (live, attenuated)
✓ Polio (Salk)
✓ Rocky mountain spotted fever
✓ Typhoid (injection)
vaccinations with no deferral (toxoids, synthetic, killed)
Hypnotics used at bedtime
Blood pressure medications
Bronchodilators
Decongestants
Oral contraceptives
Replacement hormones
Weight-reduction drugs
Mild analgesics
Vitamins
Tetracyclines and other antibiotics taken for acne
medications commonly accepted for blood donation
Copper Sulfate
what is used to estimate hemoglobin
Spectrophotometric Methods
method that determined hemoglobin when using copper sulfate
12.5 g/dL (125 g/L)
38%
minimum hemoglobin and hematocrit level for whole blood donation
125 - 175 g/L
range of hemoglobin level for female donors according to DOH-NVBSP
135 - 185 g/L
range of hemoglobin level for male donors according to DOH-NVBSP
<37.5 C (99.5 F)
body temp
< 160/100 mm Hg
blood pressure according to DOH-NVBSP
True
T or F
The medical director establishes the normal range of blood pressure for each facility and the appropriate deferral when the donor does not meet the requirement.
50-100 bpm
pulse rate according to FDA for PLASMA DONORS
None
pulse rate for whole blood donors according to AABB standards or FDA
60-100 bpm
pulse rate according to DOH-NVBSP
>50 kg (>110 lbs)
weight of donor
10.5 mL of blood/kg
max volume of blood to be collected for whole blood collection, inclusive of pilot tubes testing
Confidential Unit Exclusion (CUE)
Donors must be given a confidential way to indicate that their blood should not be used for transfusion.
Barcode Label System
can be used where the donor is given a card with two barcode labels.
CUE
Pressure from family, friends, or coworkers may cause someone to donate when he or she realizes they should not.
provides a confidential way to indicate this when more open ways may not be acceptable to the donor.
False (not always)
T or F
The person taking the donor’s health history should be the phlebotomist.
Antecubital Area
where is blood ususally drawn from the donor
vasovagal reaction
most common type of donor reaction which can cause fainting
Remove needle and tourniquet
Elevate legs above head
Apply cold compresses to forehead and back of neck
treatment when the donor experiences weakness, sweating, dizziness, pallor, nausea, and vomiting
Cold compress on back of the neck
treatment for syncope
Have donor cough
treatment for when a donor twitches or have muscle spasms
Apply pressure for 7-10 minutes
Apply ice to area for
5 minutes
treatment for hematoma
Call for help
Prevent donor from falling from the donor chair or injuring himself or herself
Ensure donor’s airway is adequate
treatment for convulsions
Begin cardiopulmonary resuscitation
Call for emergency help
treatment for cardiac difficulties
Contact the donor center if there are any concerns regarding the safety of the blood or if you believe the blood should not be transfused.
Avoid smoking for 30 minutes; avoid alcohol until something has been eaten.
Drink more fluids than usual in the next 4 hours.
If dizziness or fainting occurs, lie down or sit with the head between the knees.
Caution donors who work at certain occupations who will be returning to work immediately (involving heights, construction, or machine operators).
Remove the bandage after a few hours.
Inform the blood center if any symptoms persist.
Post-donation fluid replacement begins in the donor room.
Donors should not be released until checked by a staff member.
post-donation instructions and care
ABO group
Rh type
Weak D when needed
Antibody screening
what should the blood be tested for
Antibody identification
what should the blood undergo if positive for antibody screening
✓ Syphilis
✓ Hepatitis B
✓ Hepatitis C
✓ Human T cell lymphotropic virus (HTLV)
✓ HIV
✓ West Nile virus
Infectious disease testing currently required by the FDA on units intended for allogeneic use includes tests for ____________
• ABO/Rh
• Antibody screen
• HBsAg
• Anti-HBc
• Anti-HCV and NAT
• Anti-HIV 1/2 and NAT
• WNV RNA
• Anti-HTLV I/II
• Serologic test for syphilis
Blood donor serologic screening tests
Donor Lookback
performed whenever a repeat donor tests positive for an infectious disease when the donor was previously negative.
the blood center checks which patient(s) received the blood products from the donor’s previous donation and whether the patient(s) contracted the disease for which the donor now tests positive.
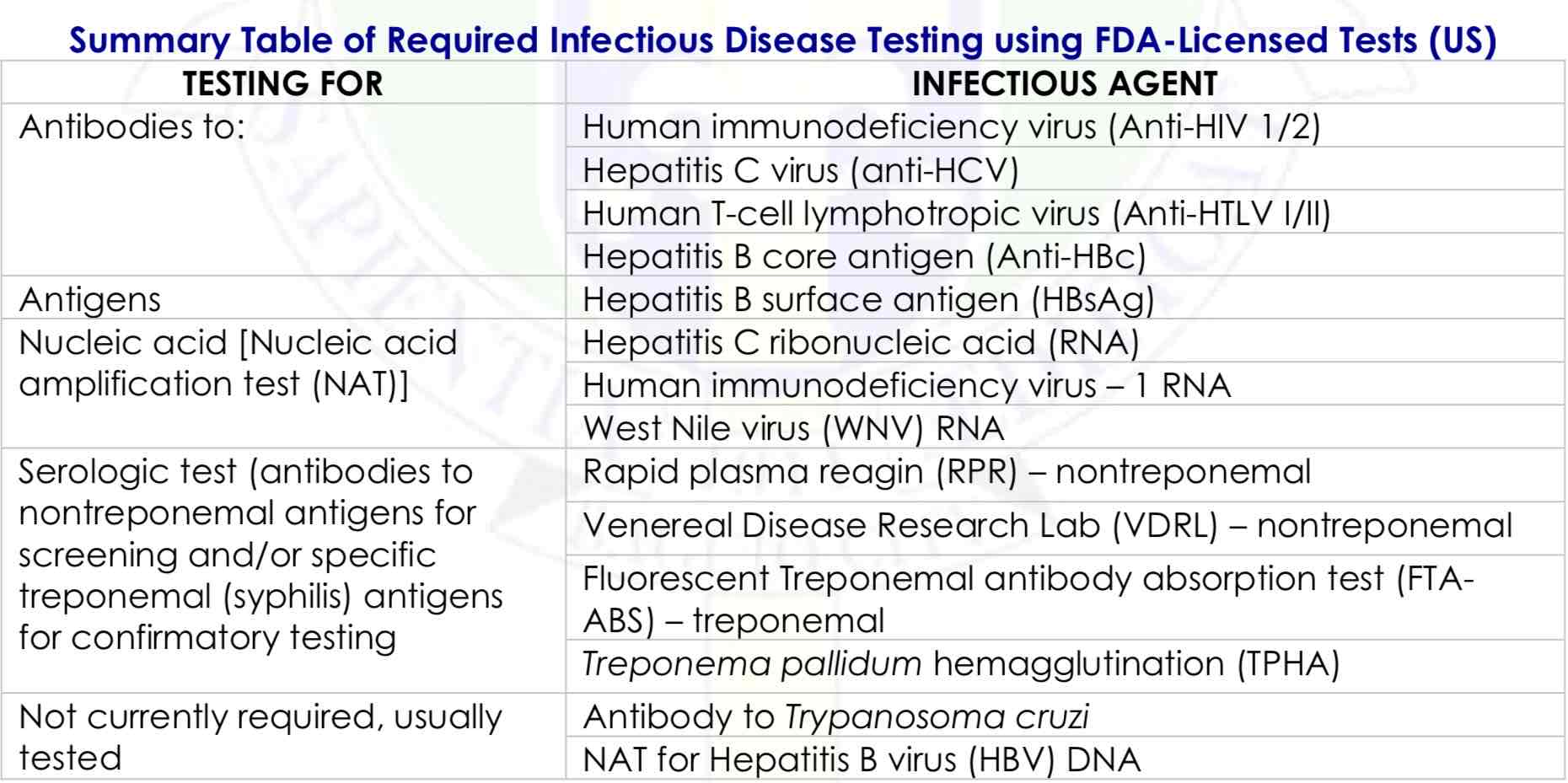
Summary Table of Required Infectious Disease Testing using FDA-Licensed Tests (US)
Autologous Donations
Recipient-Specific “Designated” or “Directed” Blood Donations
Directed/Designated Donations
Apheresis/Hemapheresis
Therapeutic Phlebotomy/Therapeutic Donation/Therapeutic Apheresis
special blood collection
Autologous Donations
one who donates blood for his or her own use; thus, such a donor is referred to as the donor-patient.
Autologous Donations
used to treat surgical blood loss in very specific situations
where there is a reasonable opportunity to avoid homologous transfusions or when
compatible allogeneic blood is not available.
Rare Blood Types
Multiple Antibodies difficult to find
autologous blood is beneficial for them
False (less stringent)
True
T or F
Medical history and physical exam requirements for autologous donation are more stringent than those for allogeneic donations.
There is no minimum or maximum age requirement in autologous donation
✓ A prescription or order from the patient’s physician.
✓Hgb conc: 11 g/dL or HCT: 33%.
✓ Collection at least 72 hours before the anticipated surgery or transfusion.
✓ Deferral for conditions presenting a risk of bacteremia.
✓ Use only for the donor-patient if labeled “autologous use only.”
criteria for autologous donations specified by the FDA, AABB
Prevent Transfusion-transmitted diseases
Prevent of alloimmunization
Supplement blood supply
Prevent febrile and allergic reactions
Reassurance of patient
advantage of autologous blood
bacterial contamination
circulatory overload
cytokine-mediated reactions
misidentification of the product or patient
possible risks in autologous donation
Inventory Control
Preoperative Anemia
Increased Cost
High Wastage
Inc Incidence of Adverse Reactions to donation
disadvantage of autologous donation
Autologous Donation
there is a high percentage of wasted units (30% to 50%) because patients end up not requiring any or all of the units donated
Unstable angina
Recent myocardial infarction or cerebrovascular accident
Significant cardiac or pulmonary disease with ongoing symptoms but without an evaluation by the treating physician
untreated aortic stenosis
Contraindications to autologous blood donation should be defined by the blood center and may include medical conditions associated with the greatest risk from blood donation, such as _________
True
T or F
The AABB Standards does not permit “crossing over” of unused autologous units into the general inventory, except in exceptional circumstances.
The decision must be approved by the medical director on a case-by-case basis.
Preoperative collection
Acute normovolemic/ isovolemic hemodilution
Intraoperative collection
Postoperative collection
methods for obtaining autologous blood
Preoperative collection
Occurs during the 5 to 6 weeks immediately preceding a scheduled,
elective surgical procedure unless the red blood cells and plasma are
scheduled to be frozen
The last blood collection should occur no later than 72 hours (3 days)
before the scheduled surgery to allow for volume replacement.
Acute normovolemic/ isovolemic hemodilution
Results in the collection of whole blood with the concurrent infusion of
crystalloid or colloid solutions, thus maintaining a normal blood volume
but decreasing the patient’s hematocrit.
Acute normovolemic/ isovolemic hemodilution
Performed in surgery immediately prior to beginning the surgical
procedure and is managed by anesthesiology.
The blood is collected in standard blood bags containing anticoagulant
or preservative and is stored in the room at room temperature.
Intraoperative collection
Involves collecting shed blood from the surgical site; processing the
blood through an instrument that washes it with saline to remove tissue
debris, free hemoglobin, and plasma that may contain activated
coagulation factors
Intraoperative collection
Residual RBCs are concentrated to a hematocrit of 50% to 60%; and then reinfusing those cells immediately.
Has been used in cardiothoracic, major orthopedic, and cardiac surgeries, in addition to vascular surgeries, such as liver transplantation
Intraoperative collection
The advantage of using intraoperative autologous blood collection is
that it may be used in cases where preoperative donation is not possible
due to the urgency of the surgery, or when the patient cannot be
scheduled for multiple preoperative donations
The blood may be stored at room temperature for up to 6 hours or at
1°C to 6°C for up to 24 hours as long as the latter temperature has begun
within 4 hours from the end of collection.
Postoperative collection
Postoperative blood salvage is collected from a drainage tube placed
at the surgical site.
It is reinfused, with or without processing, via a microaggregate filter to
screen out any debris.
Postoperative collection
Blood is characterized as being dilute, partially hemolyzed, and defibrinated.
Recommendation is that no more than 1,400 mL should be reinfused
Blood must be reinfused within 6 hours of collection or it is to be
discarded
RECIPIENT-SPECIFIC “DESIGNATED” OR “DIRECTED” BLOOD DONATIONS
EXCEPTIONAL MEDICAL NEED
Frequent donation by a specific donor for a specific patient with a medical need requires that the donor center have a procedure that typically calls for both a request from the patient’s physician and approval by the donor center’s physician.
The donor must meet all allogeneic donor selection requirements, with the exception of donation frequency, provided that they are examined and certified by a physician.
Multiple antibodies or with antibodies to high incidence antigens who requires units from donors whose red cells are negative for the corresponding antigens
An infant with neonatal alloimmune thrombocytopenia who requires antigen-negative platelets from his or her mother.
conditions for RECIPIENT-SPECIFIC “DESIGNATED” OR “DIRECTED” BLOOD DONATIONS
DIRECTED/ DESIGNATED DONATIONS
A type of practice when a person seeks to receive blood from a named donor or a donor wishes to donate blood to be transfused to a named recipient.
Directed donors must meet the same criteria as voluntary donors, and their blood can be used for other patients if not needed by the individual for whom the donations were initially intended.
DIRECTED/ DESIGNATED DONATIONS
There is a rare possibility of graft-versus-host
disease if a donor is a close relative and the
patient is immunocompromised (e.g., an infant, a
cancer patient, or a transplant patient). In this
case, an irradiated blood product is indicated.
DIRECTED/ DESIGNATED DONATIONS
Physicians may request cytomegalovirus (CMV)- negative blood components for patients who are immunocompromised and known to be CMV-negative. Leukoreduction reduces exposure to CMV because the virus is found within the WBCs. Seronegative, leuko- reduced blood products carry the least risk for infection with CMV.