Farahat Chapter 11 MCQ
0.0(0)
Card Sorting
1/40
Earn XP
Description and Tags
Electrochemistry
Last updated 4:59 AM on 6/11/25
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
41 Terms
1
New cards
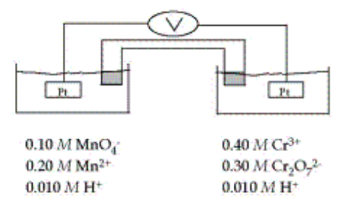
The standard reduction potentials are as follows:
MnO4–+ 8H++ 5e– → Mn2+ + 4H2O E° = 1.51 V
Cr2O72–+14H++ 6e– → 2 Cr3+ +7H2O E° = 1.33 V
What is the oxidation state of Cr in Cr2O72–?
a. +6
b. –2
c. +12
d. –1
e. +7
a. +6
2
New cards
3
New cards
4
New cards
5
New cards
6
New cards
7
New cards
8
New cards
9
New cards
10
New cards
11
New cards
12
New cards
13
New cards
14
New cards
15
New cards
16
New cards
17
New cards
18
New cards
19
New cards
20
New cards
21
New cards
22
New cards
23
New cards
24
New cards
25
New cards
26
New cards
27
New cards
28
New cards
29
New cards
30
New cards
31
New cards
32
New cards
33
New cards
34
New cards
35
New cards
36
New cards
37
New cards
38
New cards
39
New cards
40
New cards
41
New cards