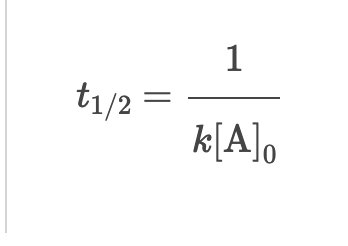Chemistry Rate Law and Elementary Steps
1/19
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
20 Terms
Elementary Step: A into Products
Molecularity: Unimolecular
Rate= k[A]
Elementary Step: A + A into Products (2A into Products)
Molecularity: Bimolecular
Rate= k[A]²
Elementary Step: A+B into Products
Molecularity= Bimolecular
Rate= k[A][B]
Elementary Step: A + A + B into Products (2A + B into Products)
Molecularity: Termolecular
Rate= k[A]²[B]
Elementary Step: A + B + C into Products
Molecularity: Termolecular
Rate=k[A][B][C]
Zero Order: Rate Law
Rate = k
Zero Order: Integrated Rate Law
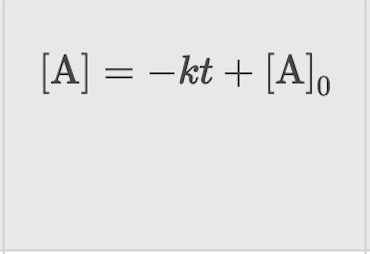
Zero Order: Plot needed to give a straight line
[A] vs. t
Zero Order: Relationship of rate constant to the slope of straight line
Slope=-k
Zero Order: Half life

First Order: Rate Law
Rate = k[A]
First Order: Integrated Rate Law
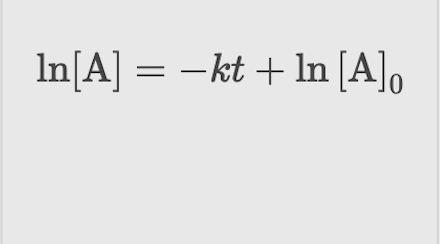
First Order: plot needed to give straight line
ln[A] vs. t
First Order: Relationship of rate constant to the slope of straight line
Slope= -k
First Order: Half Life
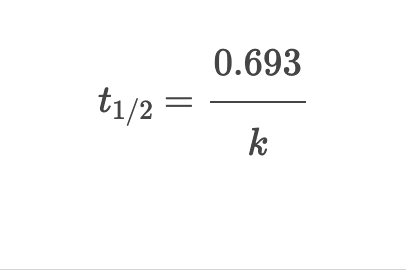
Second Order: Rate Law
Rate=k[A]²
Second Order: Integrated rate law
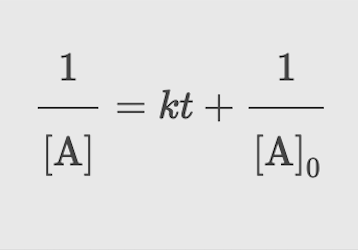
Second Order: Plot needed to give a straight line
1/[A] vs. t
Second order: Relationship of rate constant to the slope of straight line
Second Order: Half Life