BIOchem exam 3 (ALL CARDS)
1/309
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
310 Terms
Phototrophs
Obtain free energy from sunlight
Chemotrophs
obtain free energy through the oxidation of carbon fuels
mechanical work, active transport, synthesis of macromolecules
living organisms require an input of free energy to do what?
metabolism
composed of many interconnected reactions
catabolism
the breakdown of complex
molecules in living organisms to form
simpler ones, together with the release of
energy
• Usually involves oxidation
• Produces energy in biologically useful
forms (ATP and ion gradients)
Anabolism
the set of metabolic
pathways that construct bigger molecules
from smaller units.
reduced
—molecules are energy rich
oxidized
— molecules are energy poor
First stage of catabolism
Large molecules in food are broken into smaller units. This is a preparation stage; no useful energy is captured
Second stage of catabolism
Small molecules are degraded to a few simple units that play a central role in metabolism. Most of molecules are converted into acetyl CoA. Some ATP is generated (small amount)
Third stage of catabolism
ATP is produced from the complete oxidation of acetyl CoA (citric acid cycle + oxidative phosphorylation).
ATP
universal currency of free energy
Hydrolysis of ATP
Thermodynamically unfavorable reactions can be driven by favorable reactions, which is ——- in many cases
reactions must be specific, the pathway in total must be thermodynamically favorable (specific and favorable)
n order to construct a metabolic pathway, two criteria must be met:
ATP
What type of molecule is this?
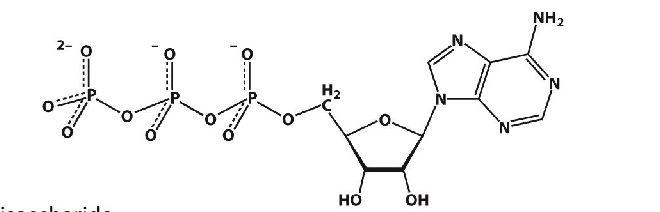
two phosphoanhydride linkages
ATP is an energy-rich molecule because its triphosphate unit has —-
higher, lower
Phosphate can be transferred from compounds with
—- ΔG’ to those with —- ΔG’.
D
Which of the following molecule(s) have a higher phosphoryl-transfer
potential than ATP?
A.Phosphoenolpyruvate
B.creatine phosphate
C.1,3-bisphosphoglycerate
D.All of the above
Phosphoenolpyruvate(PEP), creatine phosphate, 1,3- bisphosphoglycerate(1,3BPG)
What molecules have higher higher phosphoryl-transfer potential than ATP?
creatine phosphate
can regenerate ATP from ADP, allowing a short burst of activity as in a sprint
Activated carriers
small molecules carrying activated functional groups that can be donated to another molecule
ATP
Activated carrier of phosphate groups
Coenzyme A
activated carrier of two- carbon fragments (acyl groups)
NAD+ FAD
Activated carriers of electrons for fuel oxidation
NADPH
Activated carriers of electrons for the synthesis of biomolecules
Fuels
Reduced organic compounds serve as —-from which electrons can be stripped off during oxidation.
NADP+ and NAD+
The reactive site is the same in —-
NAD and NADP
Common redox cofactors
These are commonly called pyridine nucleotides.
• They can dissociate from the enzyme after the
reaction.
• In a typical biological oxidation reaction, hydride
from an alcohol is transferred to NAD+, giving
NADH.
Acetyl CoA
an important donor of acyl groups.
• feeding two-carbon units into
metabolic pathways
• synthesis of fatty acids
(HS is the reactive group)
Stage 1 of glycolysis
(preparatory stage) traps glucose in the cell and modifies it so that it can be cleaved into a pair of phosphorylated 3-carbon compounds
Stage 2 of glycolysis
oxidizes the 3-carbon compounds to pyruvate while generating 2 molecules of ATP
cytosol, phosphorylated
All 10 glycolytic enzymes are in the —-. all 10 intermediates are —— compounds of six or three carbons
Glycolysis
a universal process. One molecule of glucose is oxidized to 2 molecules of pyruvate. Useful biological energy conserved as 2 molecules of ATP and 2 of NADH
b
Glucose is a hydrophilic molecule that enters cells through glucose transporters,
from extracellular space where concentration of glucose is high into cytoplasm
where concentration of glucose is low.
What type of transport is this?
A.Simple diffusion
B.Facilitated diffusion
C.Simport
D.Antiport
E.Primary active transport
Step one: phosphorylation of glucose
reaction is irreversible inside cell
Glucose — hexokinase— Glucose 6- Phosphate
glucose
Starting material for step1 Phosphorylation of glucose
hexokinase
Enzyme for step1 Phosphorylation of glucose
Glucose-6 phosphate
Final product of step1 Phosphorylation of glucose
two lobes separated
Hexokinase in the absence of glucose
two lobes of the enzyme come together
Hexokinase after the binding of glucose
b
How glucose is different from fructose?
A. Glucose is a hexose, fructose is a pentose
B. Glucose is an aldose, fructose is a ketose
C. Glucose major form is cyclic, fructose is mostly linear
D. Glucose is predominantly in a D-form, fructose is predominantly in L-form
E. Glucose can be used as a fuel in our body, fructose cannot
Step 2: Phosphohexose Isomerization
this reaction proceeds in either direction
Glucose 6- phosphate —- phosphohexose isomerase — Fructose 6-phosphate
glucose-6 phosphate
Starting product of Step 2: Phosphohexose Isomerization
phosphohexose isomerase
enzyme of Step 2: Phosphohexose Isomerization
fructose 6-phosphate
final product of Step 2: Phosphohexose Isomerization
Step 3: 2nd Priming Phosphorylation
First committed step of glycolysis; second irreversible step
activity increased when ATP is low, and/or ADP and AMP
are high;
• Enzyme is inhibited when cell has plenty of ATP
Fructose 6-phosphate- phosphofructokinase-1(PFK-1)—Fructose 1,6-biphosphate
Fructose 6-phosphate
starting material of Step 3: 2nd Priming Phosphorylation
phosphofructokinase-1 (PFK-1)
enzyme of Step 3: 2nd Priming Phosphorylation
fructose 1,6-biphosphate
final product of Step 3: 2nd Priming Phosphorylation
Step 4: Aldol Cleavage of F-1,6-bP
This reaction is thermodynamically unfavorable/reversible.
Product (GAP) concentration is kept low to pull reaction forward.
fructose 1,6-biphosphate—aldolase— dihydroxyacetone phosphate and glyceraldehyde 3-phosphate
fructose 1,6-biphosphate
Starting material of Step 4: Aldol Cleavage of F-1,6-bP (this gets cut in half)
aldolase
enzyme of Step 4: Aldol Cleavage of F-1,6-bP
dihydroxyacetone phosphate and glyceraldehyde 3-phosphate
final products of Step 4: Aldol Cleavage of F-1,6-bP
Step 5: Triose Phosphate Interconversion
Dihydroxyacetone phosphate— triose phosphate isomerase— glyceraldehyde 3-phosphate
dihydroxyacetone phosphate
Starting material of Step 5: Triose Phosphate Interconversion
triose phosphate isomerase
enzyme for Step 5: Triose Phosphate Interconversion
glyceraldehyde 3-phosphate
final product of Step 5: Triose Phosphate Interconversion
payoff phase
The energy gain comes from the —- of glycolysis (steps 6-10)
glucose carbons in GAP
fructose 1,6-biphosphate, dihydroxyacetone phosphate, glyceraldehyde 3-phosphate
Step 6: Oxidation of GAP by Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH)
First energy conserving reaction of glycolysis
Carbon 1 is oxidized
• A high-energy phosphate
compound generated
• Inorganic phosphate is
incorporated
• Oxidation of aldehyde with
NAD+ gives NADH
• The amount of NAD+ in a cell is
far smaller than the amount of
glucose metabolized in a few
minutes. NADH formed in this
step should be continuously
reoxidized and recycled.
Glyceraldehyde 3-phopshate and inorganic phosphate
Starting material for Step 6: Oxidation of GAP by
Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH)
Glyceraldehyde 3-phosphate dehydrogenase
enzyme for Step 6: Oxidation of GAP by Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH)
1,3-biphosphoglycerate (1,3 BPG)
Final product of Step 6: Oxidation of GAP by Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH)
Step 7: 1st Production of ATP
Makes 1 ATP
1,3-bisphosphoglycerate —-Phosphoglycerate kinase—-ATP and 3-phosphoglycerate
1,3-bisphosphoglycerate (1,3 BPG)
starting material of Step 7: 1st Production of ATP
a high-energy compound. It can donate the phosphate group to ADP to make ATP
Phosphoglycerate kinase
Enzyme for Step 7: 1st production of ATP
is named for the reverse reaction. Like all enzymes, it catalyzes the reaction in both directions. Reverse reaction takes place in gluconeogenesis.
ATP and 3-phosphoglycerate
Final products of Step 7:1st production of ATP
Step 8: Migration of the Phosphate
3-phosphoglycerate—Phosphoglycerate mutase— 2-phosphoglycerate
2,3 BPG formed in this step
3- phosphoglycerate
starting material for Step 8: Migration of the Phosphate
phosphoglycerate mutase
enzyme for Step 8: Migration of the Phosphate
2-phosphoglycerate
final product of Step 8: Migration of the Phosphate
Step 9: Dehydration of 2-PG to PEP
A high energy phosphate compound (PEP) generated
2-phosphoglycerate— enolase— phosphoenolpyruvate
2-phosphoglycerate
starting product of Step 9: Dehydration of 2-PG to PEP
enolase
Enzyme for Step 9: Dehydration of 2-PG to PEP for
phosphoenolpyruvate(PEP)
Final product of Step 9: Dehydration of 2-PG to PEP
Step 10: 2nd Production of ATP
• 2nd substrate-level phosphorylation of
glycolysis
• Pyruvate kinase requires K+ and
Mg++ or Mn++ for activity
• Loss of phosphate from PEP yields an
enol that tautomerizes into ketone
(see next slide)
• Tautomerization effectively lowers the
concentration of the reaction product
phosphoenolpyruvate— pyruvate kinase— ATP and pyruvate
phosphoenolpyruvate(PEP)
Starting material for Step 10: 2nd Production of ATP
Pyruvate kinase
Enzyme for Step 10: 2nd Production of ATP
Pyruvate and ATP
Ending material for Step 10: 2nd Production of ATP
Pyruvate tautomerization
— drives ATP production
drives the reaction toward ATP formation by lowering the concentration of the reaction product (enol form of pyruvate)
b
What is substrate-level phosphorylation?
A. phosphorylation of AMP by ATP
B. ATP synthesis when the phosphate donor is a
substrate with high phosphoryl transfer potential
C. phosphorylation of glycolytic intermediates
D. phosphorylation of ATP coupled to an ion gradient
E. ATP and AMP synthesis from two molecules of ADP
1 glucose, 2 ATP, and 2 NAD+
Glycolysis uses
2 pyruvate, 4 ATP, 2 NADH
glycolysis makes
A
Where does glycolysis take place in the cell?
A. Cytoplasm
B. Mitochondria
C. Bloodstream outside the cells
D. Nucleus
E. Endoplasmic reticulum
Lactic acid fermentation
pyruvate —lactate dehydrogenase—L-lactate
pyruvate
starting material for lactic acid fermentation
lactate dehydrogenase
Enzyme for lactic acid fermentation
l-lactate
final product of lactic acid fermentation
this is dumped out of the cell so the reaction direction is maintained
Ethanol fermentation
Two-step reduction of pyruvate to ethanol
• Humans do not have pyruvate decarboxylase.
• We do express alcohol dehydrogenase for ethanol metabolism, but is largely used in
the reverse reaction.
• CO2 produced in the first step is responsible for:
– carbonation in beer
– dough rising when baking bread
NAD+
The substance that must be regenerated for glycolysis to proceed is ____
glycolysis
Only a small amount of energy available in glucose is captured in —
cellular respiration
is the complete oxidation of organic
fuels to CO2 and H2O in the presence of O2 .
• Cells consume O2 and produce CO2 in this process
• It provides more energy (ATP) from glucose than
glycolysis
• Occurs in three major stages:
1. acetyl CoA production (Chapter 18)
2. citric acid cycle: acetyl CoA oxidation (Chapter 19)
3. electron transfer and oxidative phosphorylation
(Chapters 20-21)
Acetyl CoA
fuel for the citric acid cycle
aerobic
under — conditions pyruvate enters the mitochondria where it is converted into acetyl CoA
mitochondrial matrix
citric acid cycles occurs in the —
inner mitochondrial membrane
oxidative phosphorylation occurs in the —-
pyruvate dehydrogenase complex
catalyst for the conversion of pyruvate to acetyl-CoA
TPP, Lipollysine, FAD, NAD+, CoA-SH
5 co-enzymes required for conversion of pyruvate to Acetyl-CoA
conversion of pyruvate of acetyl-CoA
Net reaction:
– oxidative decarboxylation of pyruvate
– first carbons of glucose to be fully oxidized
• Catalyzed by the pyruvate dehydrogenase complex
– requires 5 coenzymes
– TPP, lipoyllysine, and FAD are prosthetic groups.
– NAD+ and CoA-SH are co-substrates.
prosthetic groups
TPP, Lipoyllysine , FAD