Enthalpy of Solution
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
10 Terms
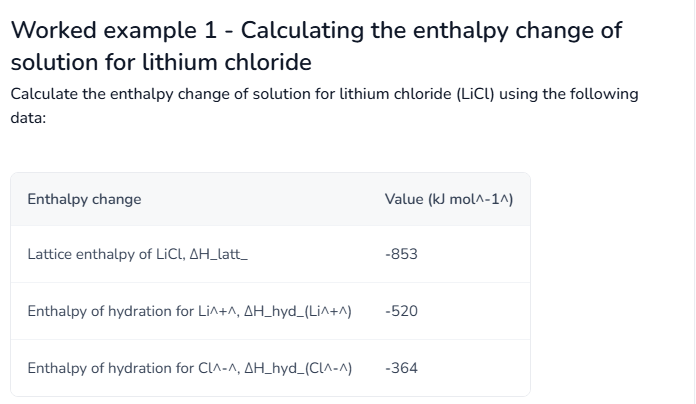
What is the standard definition of the Enthalpy of Solution (ΔHₛₒₗ)?
The enthalpy change when one mole of an ionic substance dissolves in water to form an infinitely dilute solution.
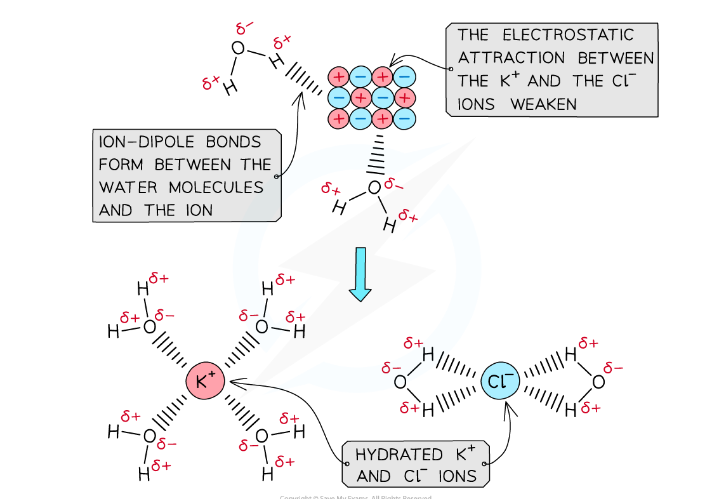
What is the standard definition of the Enthalpy of Hydration (ΔHₕyᵈ)?
The enthalpy change when one mole of gaseous ions is dissolved in water to form an infinitely dilute solution. It is always exothermic.
Why is the enthalpy of hydration always exothermic?
Because strong ion-dipole bonds are formed between the ions and the polar water molecules, which releases energy.This energy release leads to a decrease in enthalpy, making the process exothermic.
How does ionic charge affect the enthalpy of hydration?
Higher ionic charge leads to a more exothermic (more negative) ΔHₕyᵈ due to stronger ion-dipole attractions.
How does ionic radius affect the enthalpy of hydration?
Smaller ionic radius leads to a more exothermic (more negative) ΔHₕyᵈ because the charge density is higher, allowing for closer, stronger attractions to water molecules.
Which ion would have a more exothermic hydration enthalpy, Mg²⁺ or Na⁺? Explain why.
Mg²⁺. It has a higher charge (+2 vs +1), leading to stronger ion-dipole attractions with water molecules.
Which ion would have a more exothermic hydration enthalpy, Li⁺ or K⁺? Explain why.
Li⁺. It has a smaller ionic radius than K⁺, so its charge is more concentrated, resulting in stronger ion-dipole attractions.
What is the equation linking enthalpy of solution, hydration and reverse lattice enthalpy?
ΔHhydꝋ = ΔHlattꝋ + ΔHsolꝋ